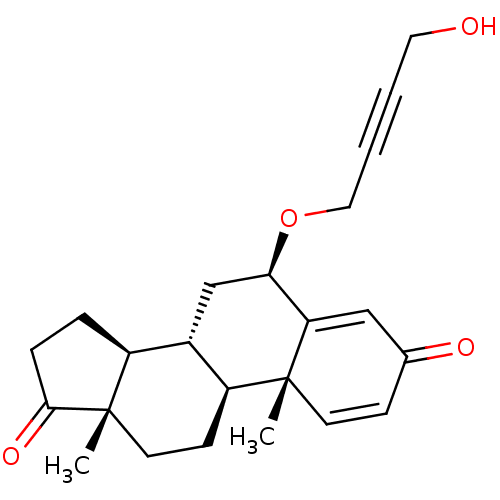
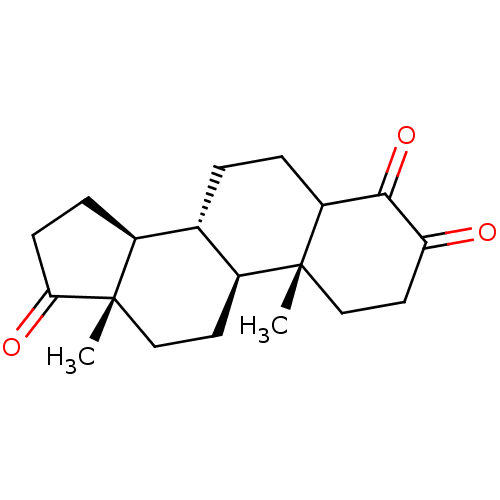
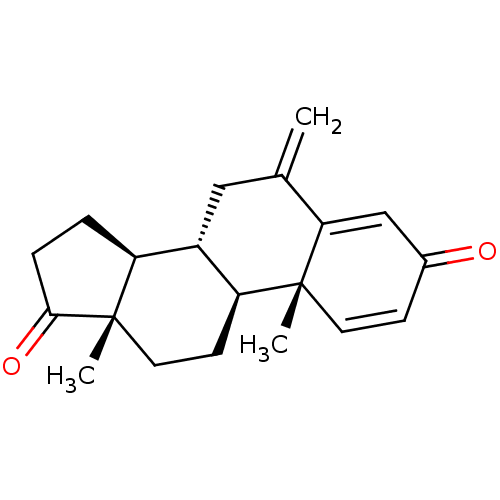
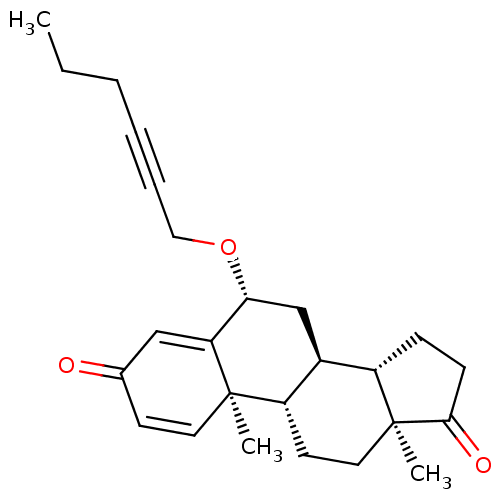
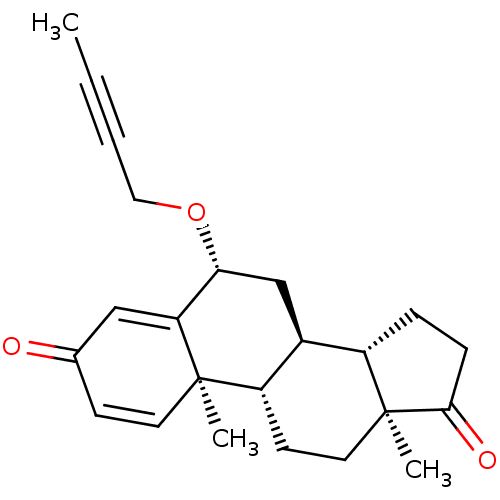
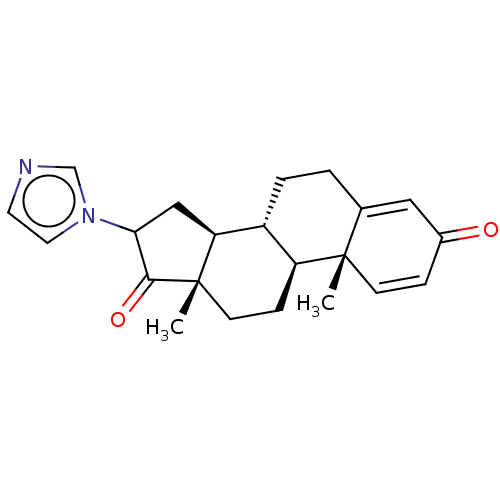
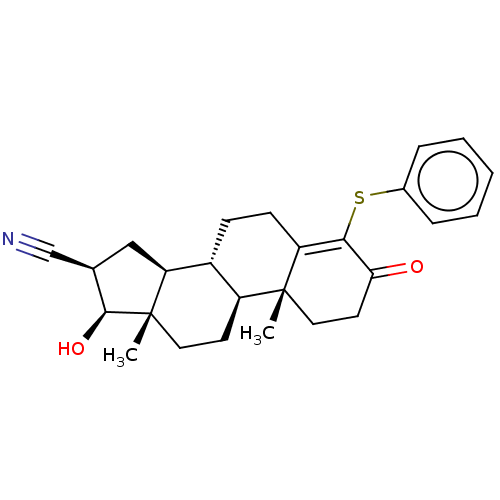
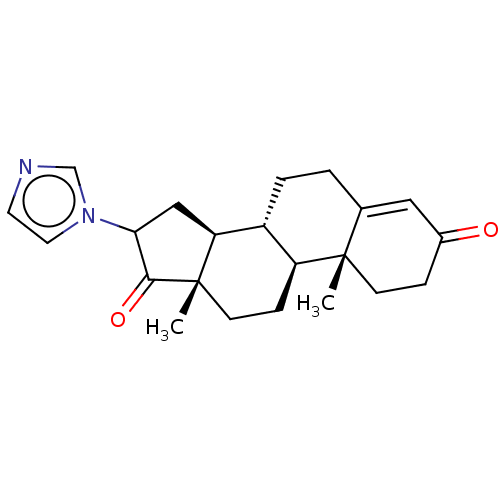
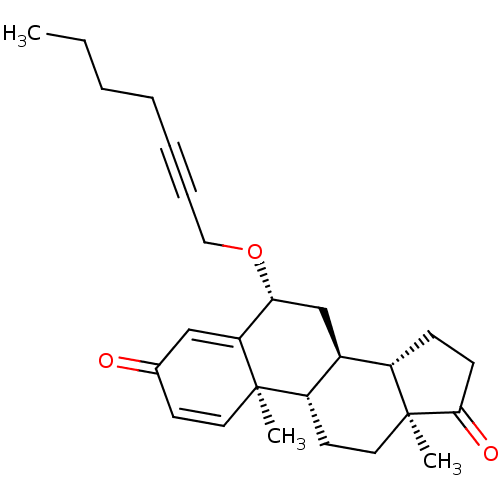
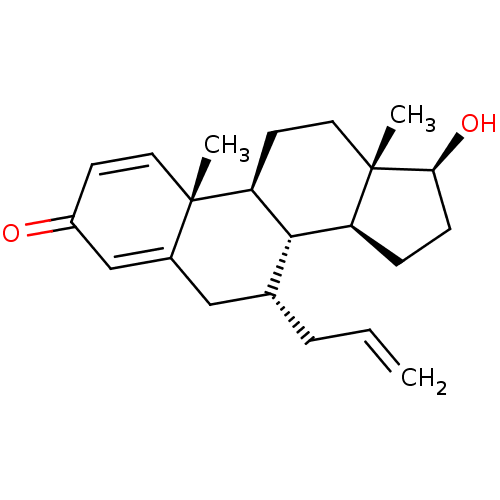
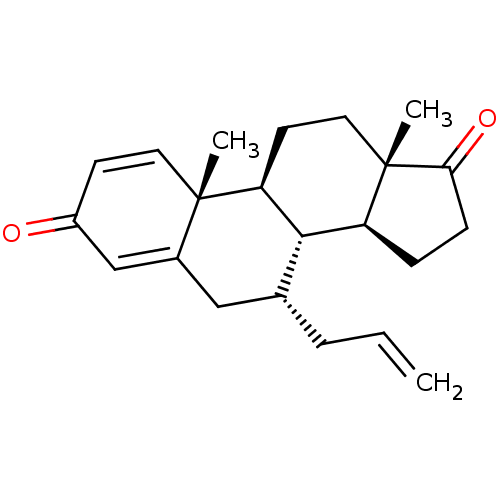
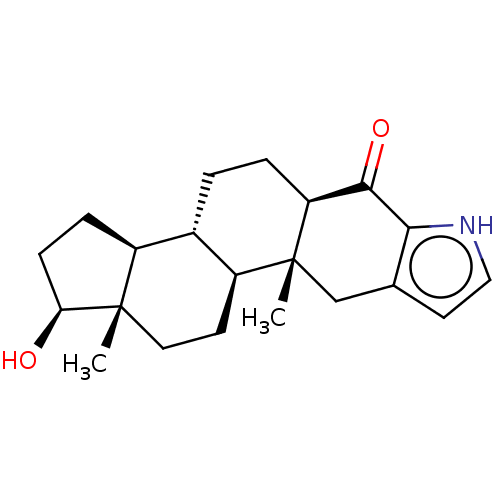
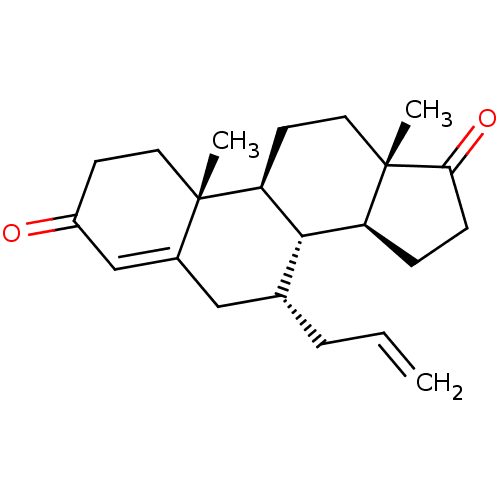
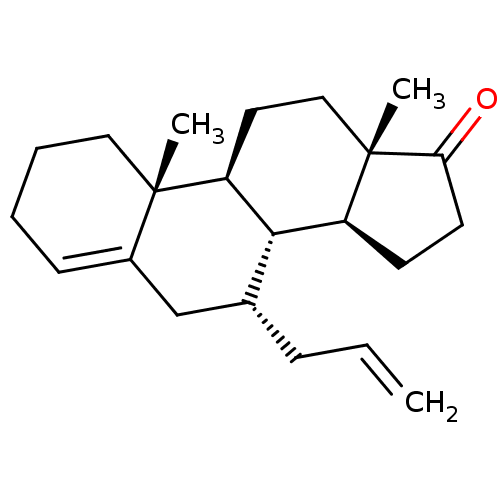
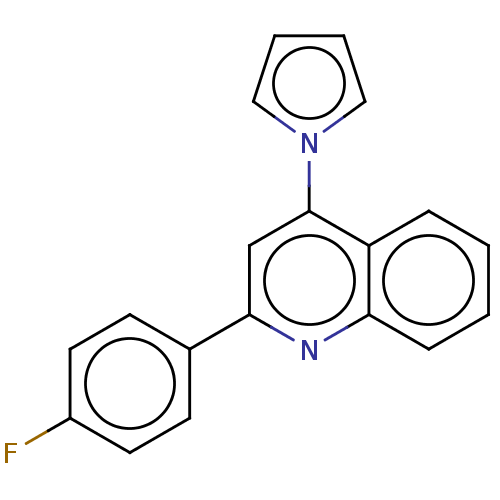
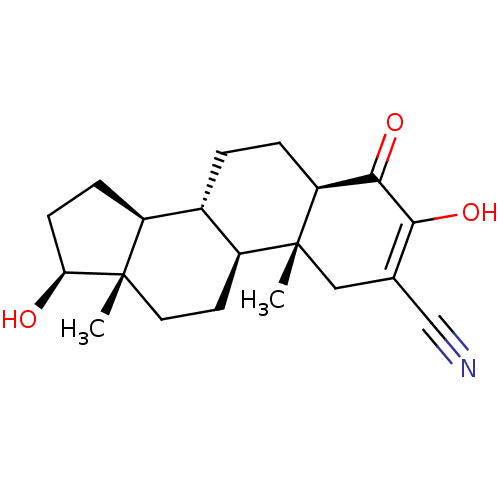
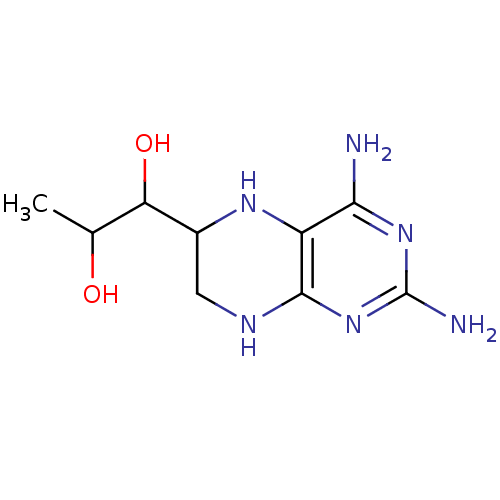
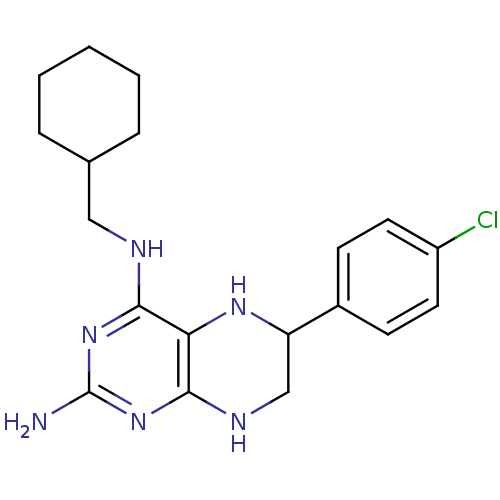
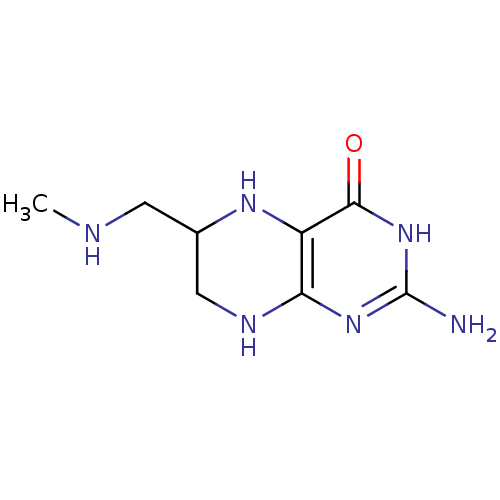
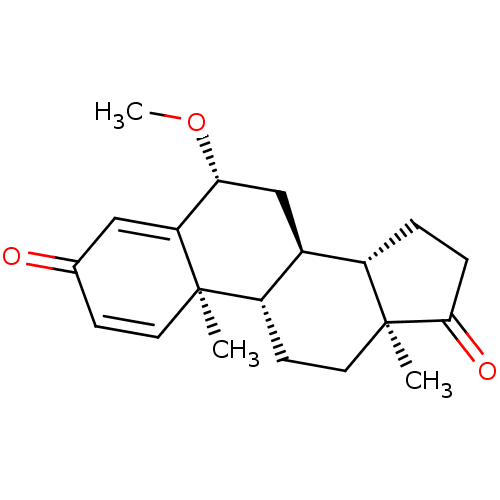
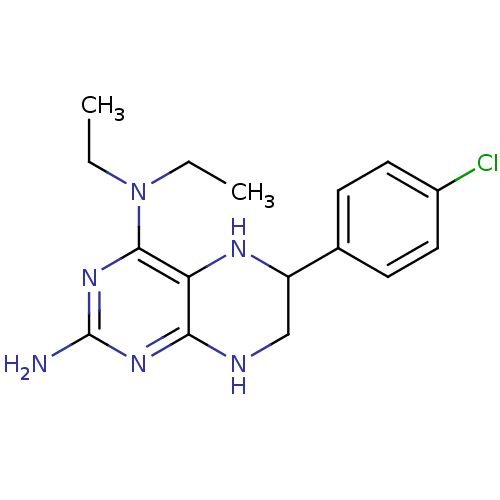
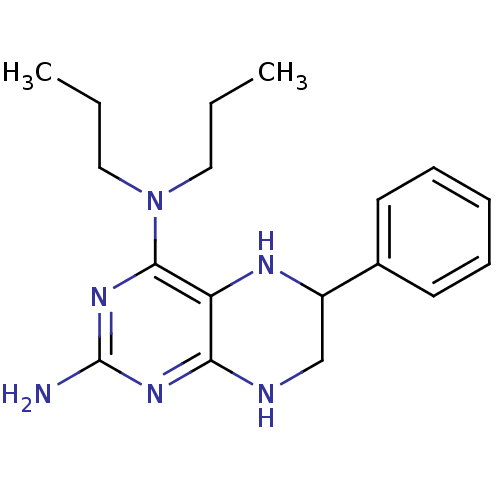
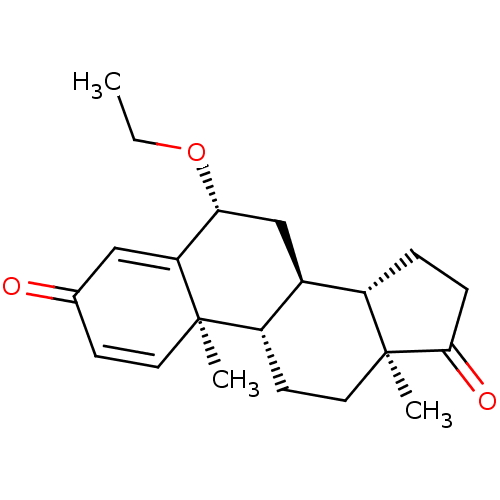
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataKi: 84nMAssay Description:Competitive inhibition of human placental microsomal aromatase using androgen as substrateMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 0.00400nMAssay Description:Inhibition of aromatase (unknown origin) transfected in human MCF7 cellsMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of aromatase in human JEG3 cells using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 1 hr by scintillati...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of aromatase in human JEG3 cells using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 1 hr by scintillati...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Reversible inhibition of aromatase (unknown origin)More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of aromatase (unknown origin) transfected in human MCF7 cellsMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 9.90nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 11.8nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 minsMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of particulate fractions of human breast cancer derived aromataseMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 48.6nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 50.1nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 112nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 168nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 169nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 5 mins by be...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 181nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 512nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l...More data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of human aromatase using testosterone as substrate incubated for 1 hr by HTRF assayMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 1.02E+3nMAssay Description:Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5...More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-II with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 2.18E+3nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.68E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 4.12E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-III with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.46E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-III with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 9.28E+3nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human aromatase transfected in human MCF7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-III with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-II with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.47E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-III with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
Affinity DataIC50: 1.57E+4nMAssay Description:Inhibitory activity against human Nitric oxide synthase-I with 2 uM H4Bip for 30 minMore data for this Ligand-Target Pair
TargetAromatase(Homo sapiens (Human))
State University of New York Upstate Medical University
Curated by ChEMBL
State University of New York Upstate Medical University
Curated by ChEMBL
Affinity DataIC50: 1.81E+4nMAssay Description:Inhibition of human placental aromatase using [3H]-1beta-androstenedione as substrate after 16 hrs by [3H]-water methodMore data for this Ligand-Target Pair


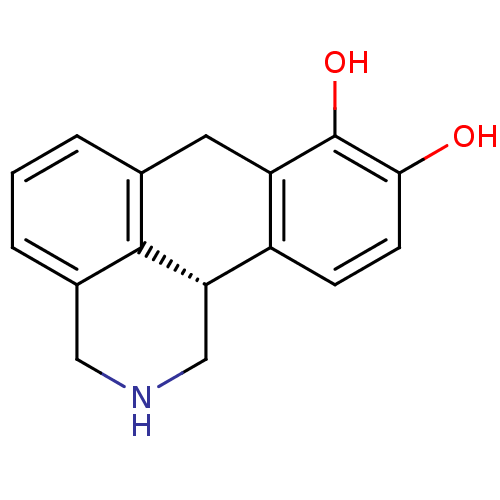

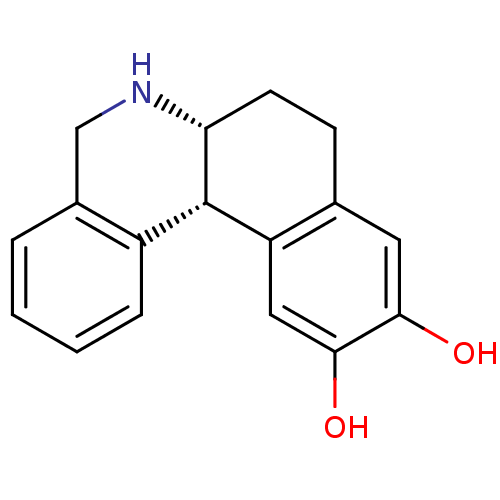
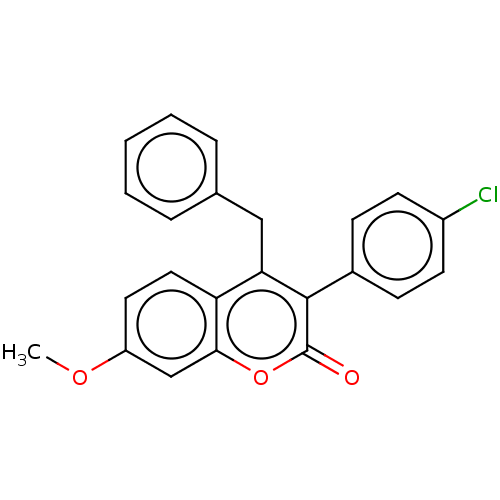

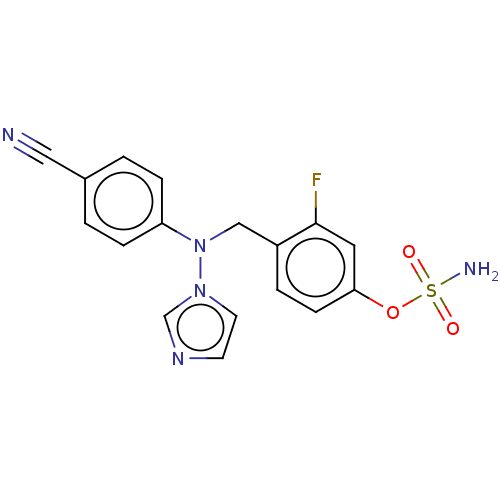
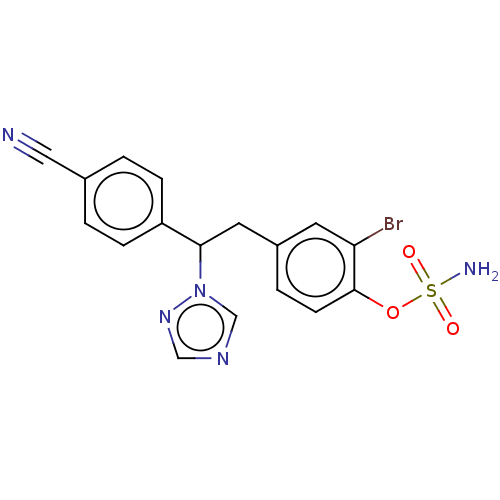
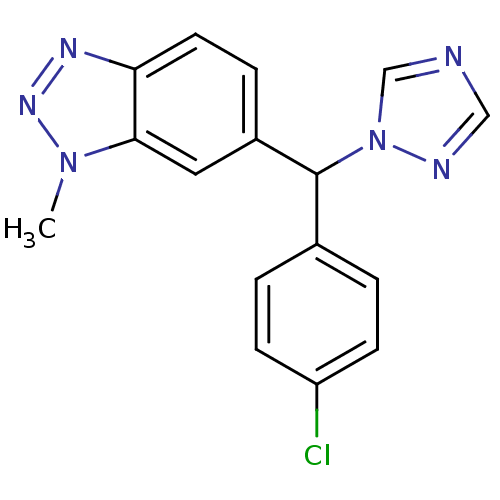
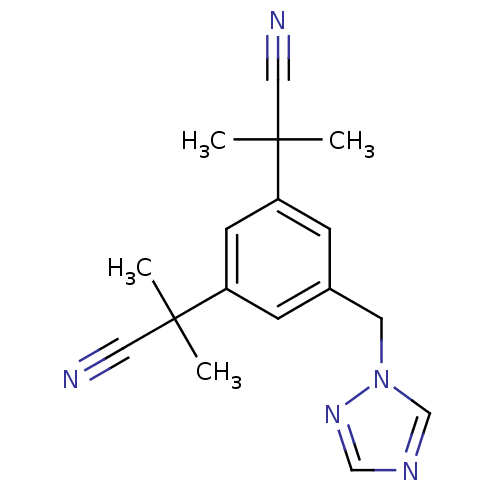

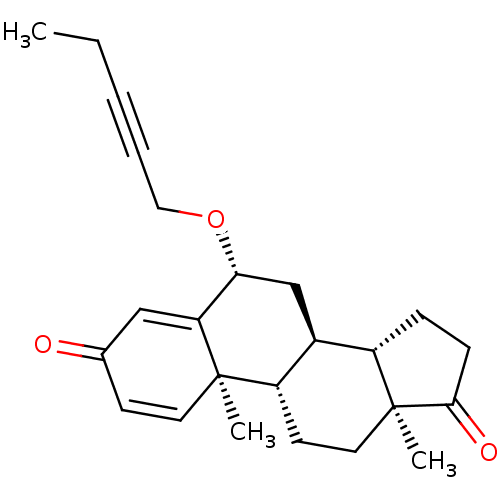
 3D Structure (crystal)
3D Structure (crystal)