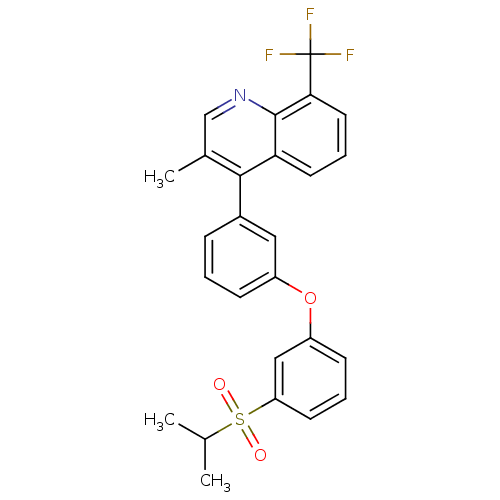
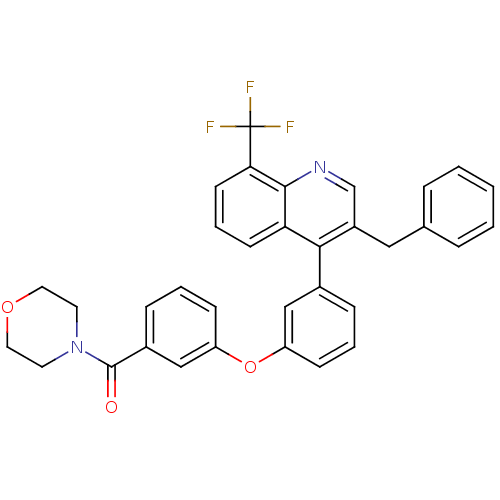
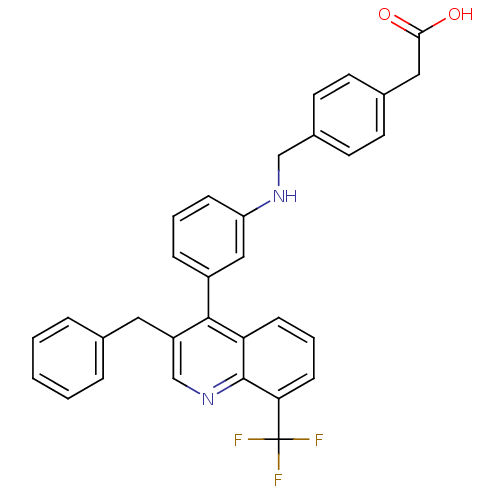
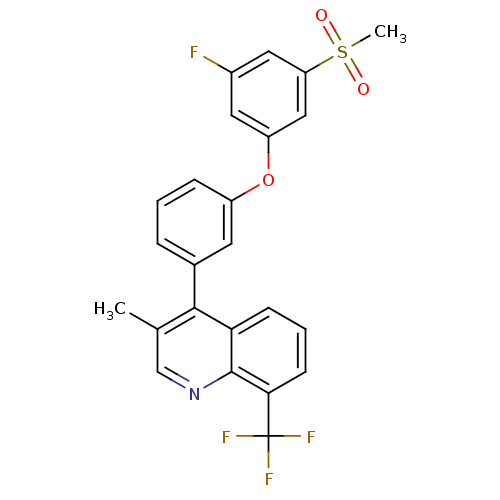
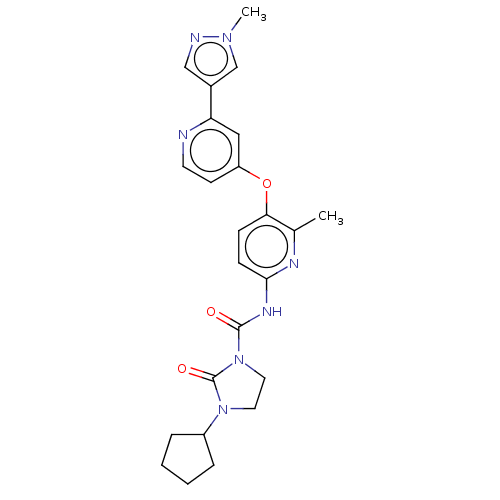
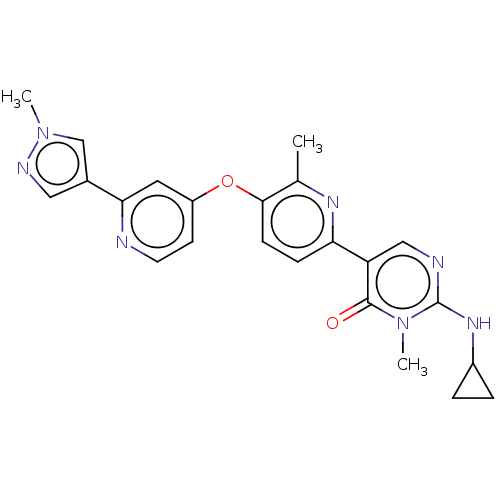
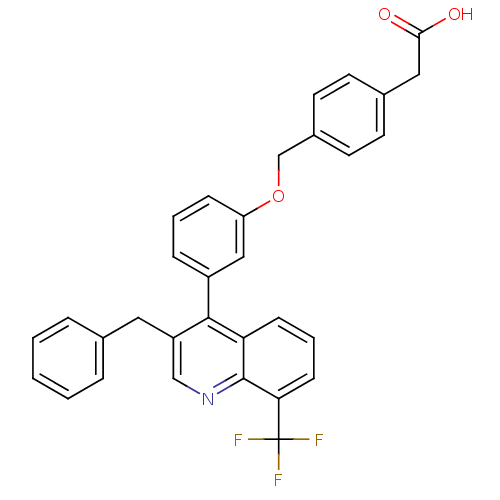
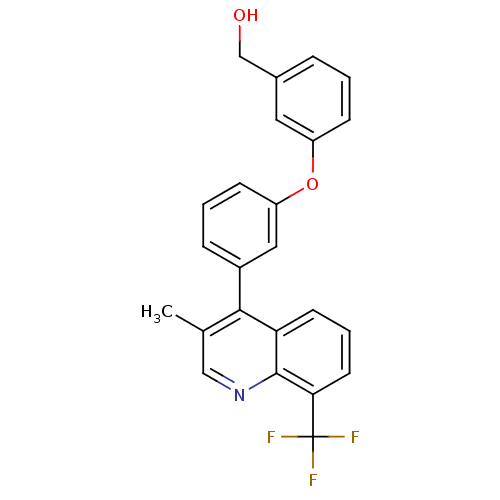
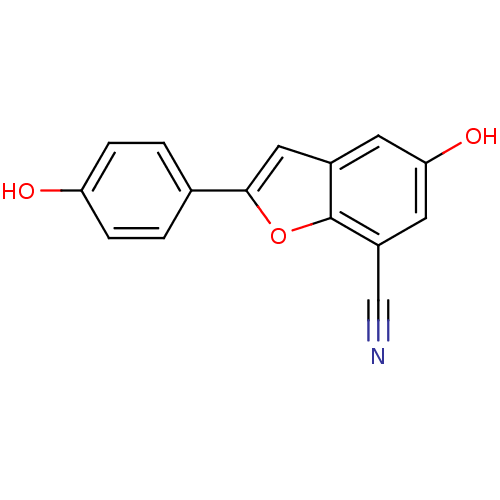
Affinity DataIC50: 0.350nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
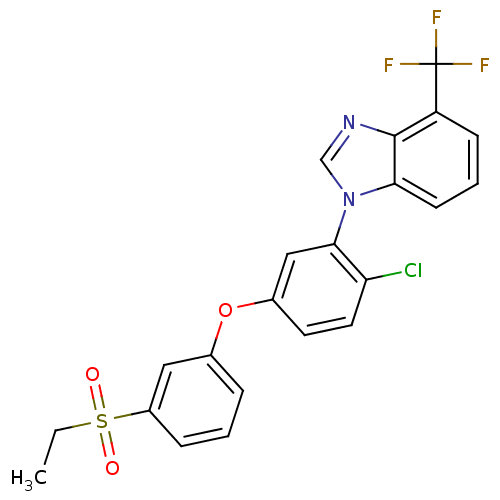
Affinity DataIC50: 0.460nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of MEK assessed as inhibition of ERK phosphorylation by Raf-MEK-ERK cascade assayMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of wild type CRAF (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase coupled assay in ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nM EC50: 316nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nM EC50: 23nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Binding affinity for human Estrogen receptor alphaMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of wild type CRAF (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase coupled assay in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 138nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 33nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta-LBDMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor [538-972](Homo sapiens (Human))
Deciphera Pharmaceuticals, LLC
US Patent
Deciphera Pharmaceuticals, LLC
US Patent
Affinity DataIC50: 2nMpH: 7.5 T: 2°CAssay Description:Activity of unphosphorylated c-FMS kinase (uFMS, Seq. ID no. 1) was determined by following the production of ADP from the FMS kinase reaction with A...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Eli Lilly and Company
Curated by ChEMBL
Eli Lilly and Company
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant BRAF V600E mutant (unknown origin) assessed as ADP formation measured for 5 hrs by pyruvate kinase/lactate dehydrogenase co...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1/2(Homo sapiens (Human))
University of Auckland
Curated by ChEMBL
University of Auckland
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISAMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10nM EC50: 93nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 233nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 71nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Binding affinity for human Estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta-LBDMore data for this Ligand-Target Pair

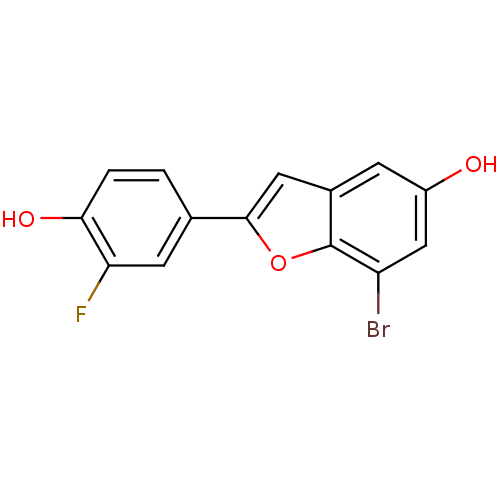
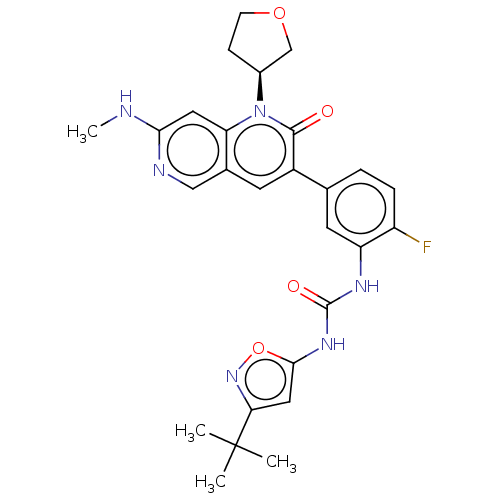
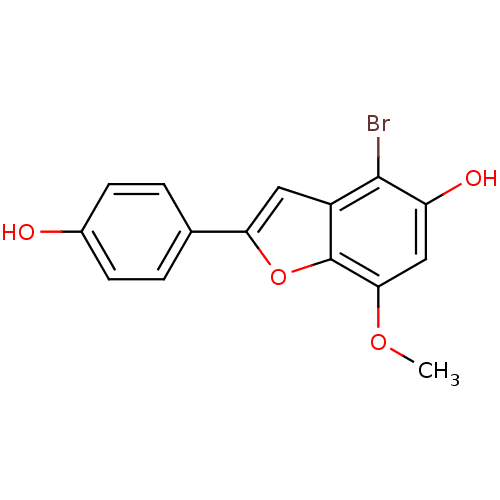

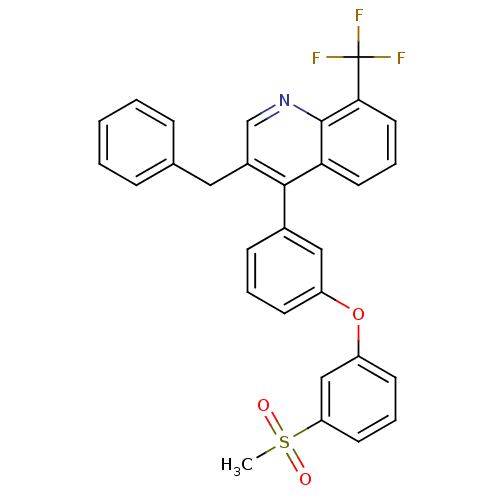

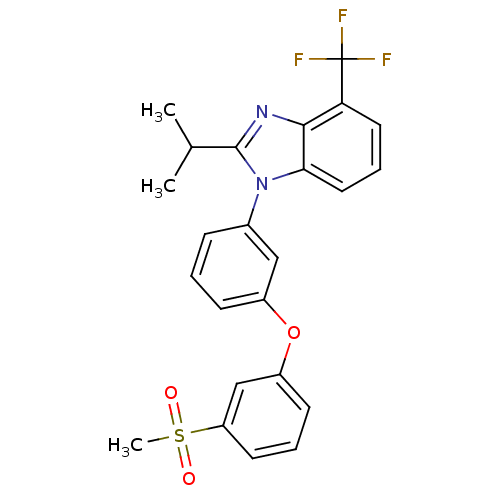

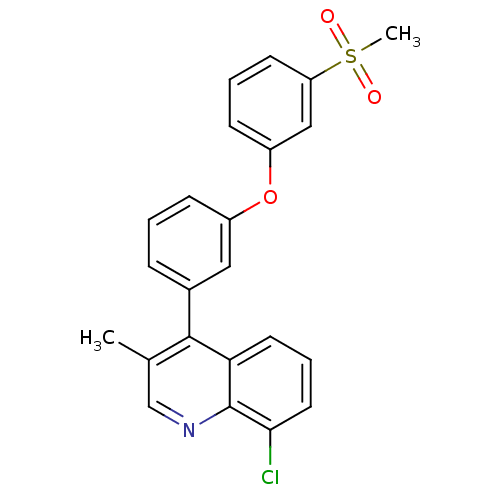
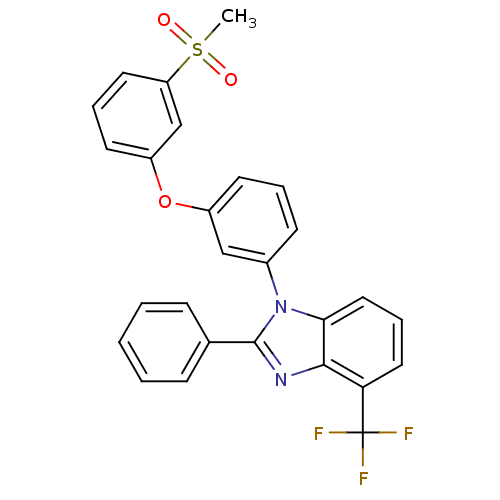
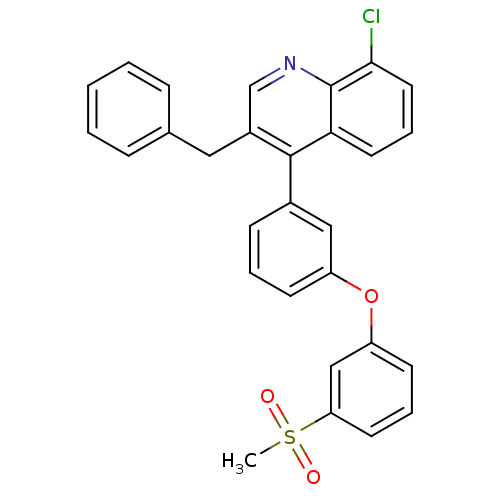
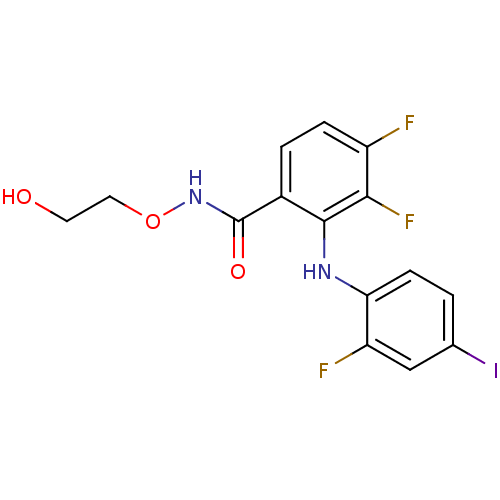
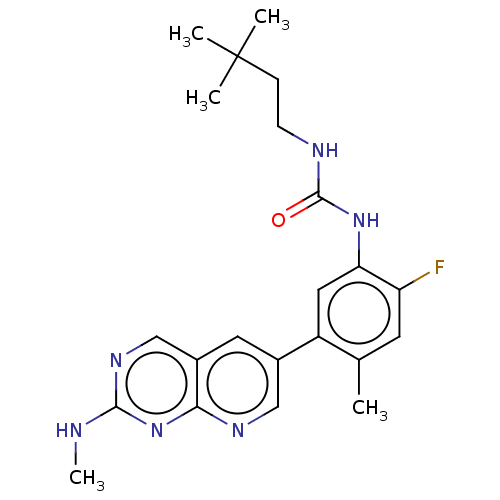
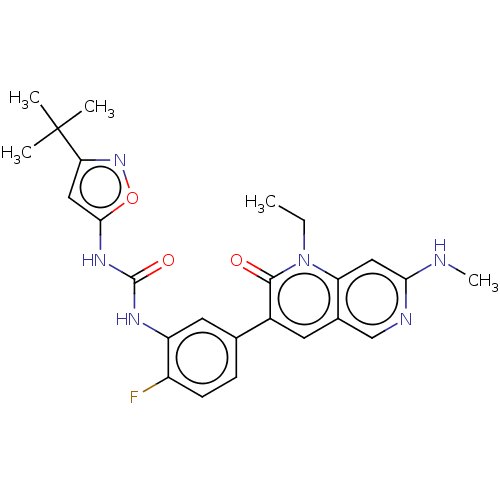

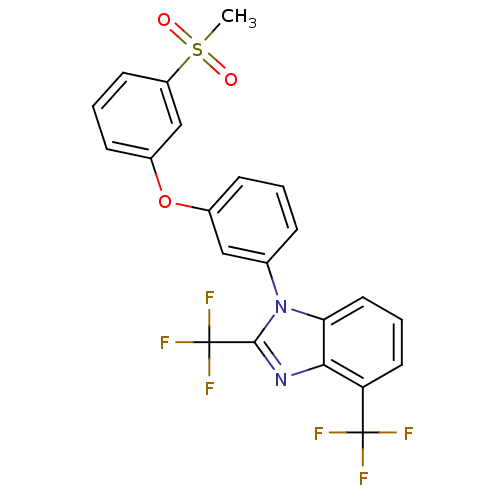
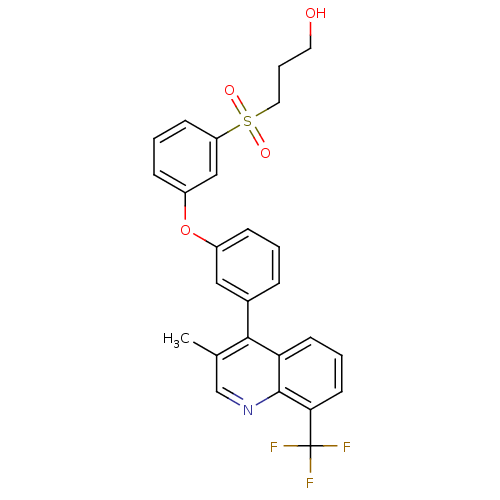
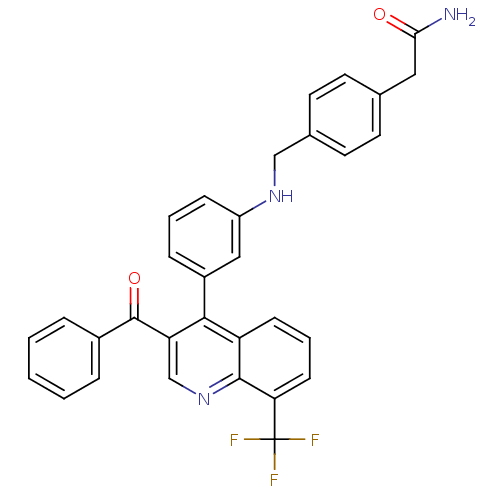

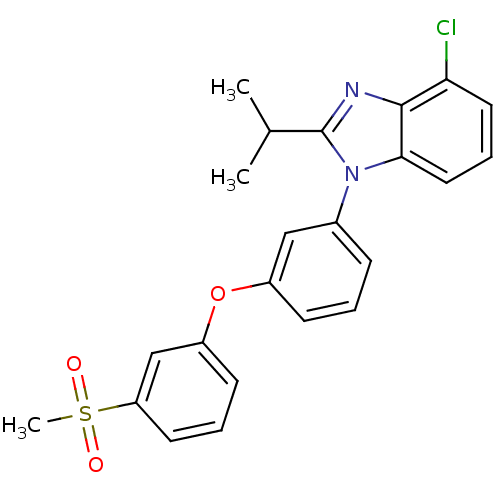
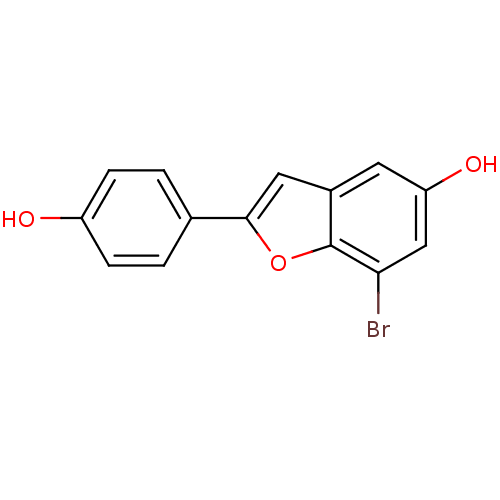
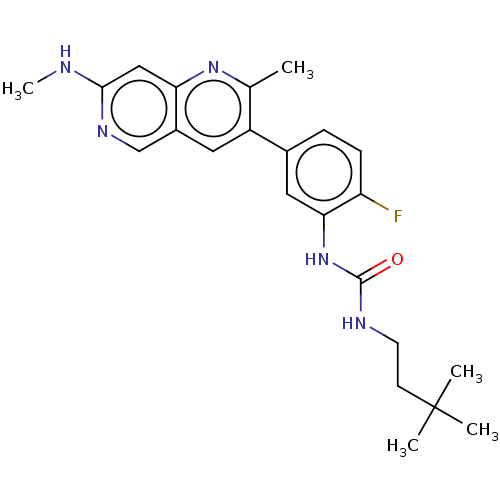



 3D Structure (crystal)
3D Structure (crystal)