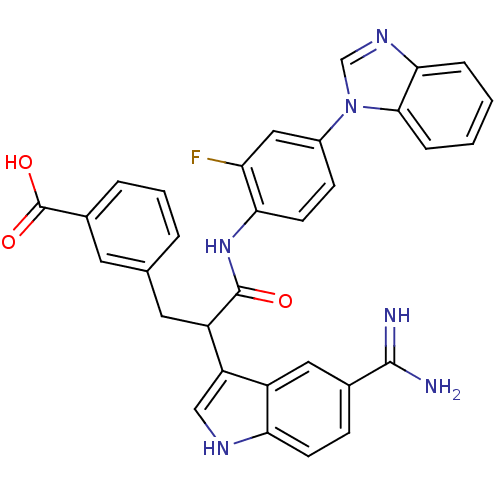
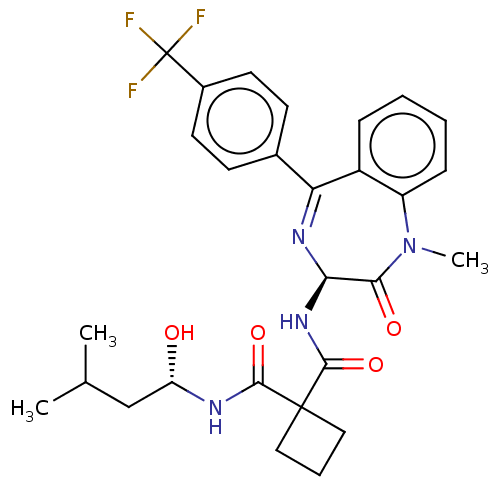
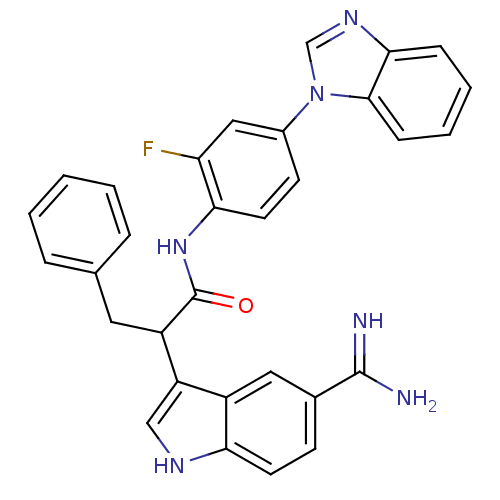
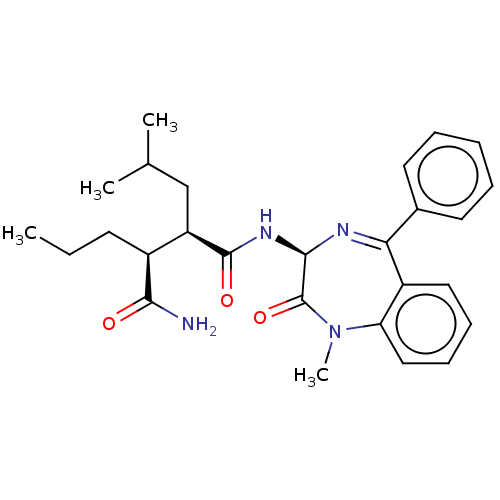
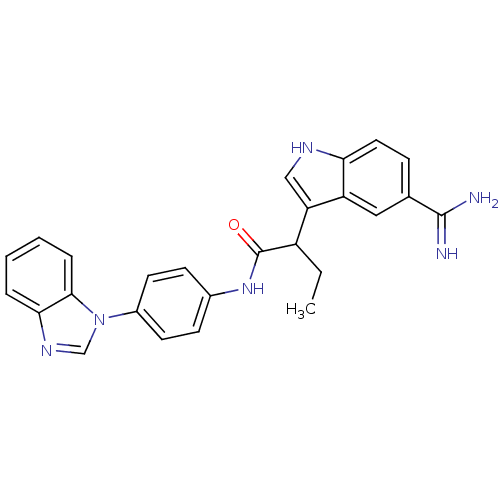
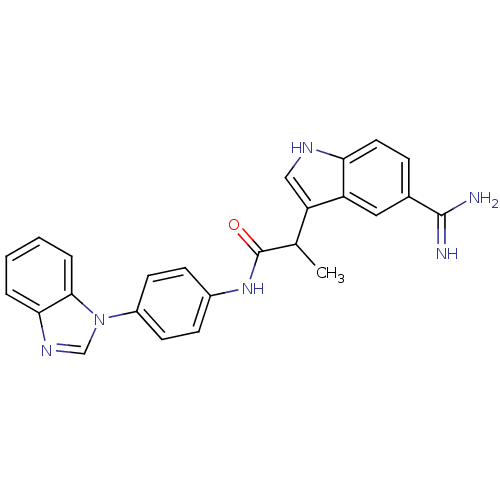
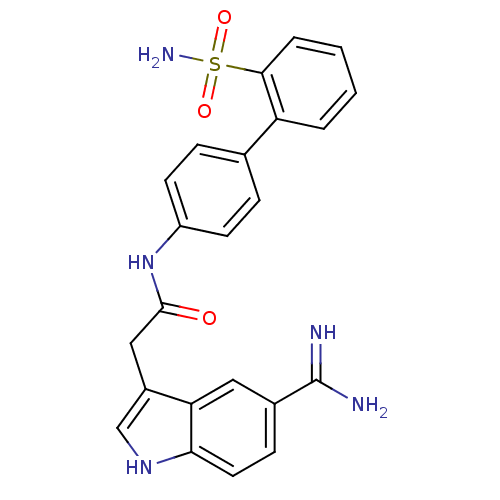
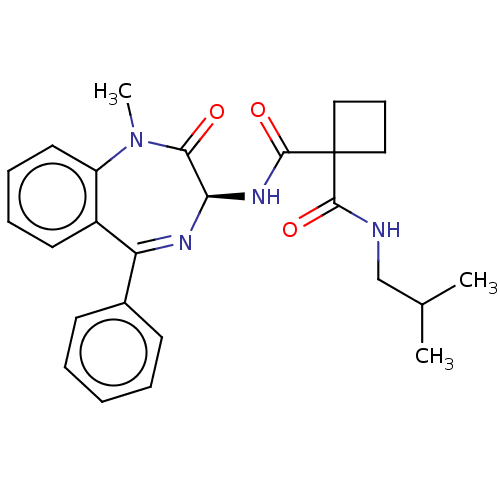
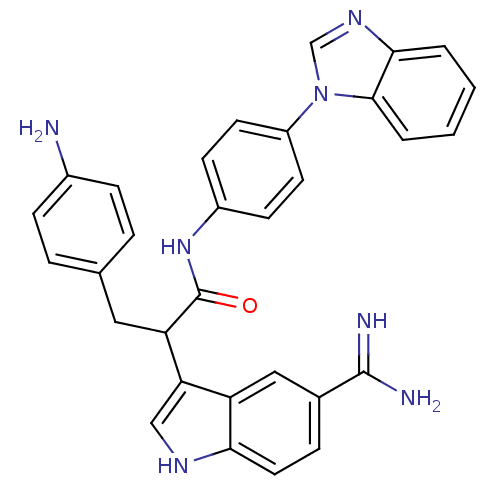
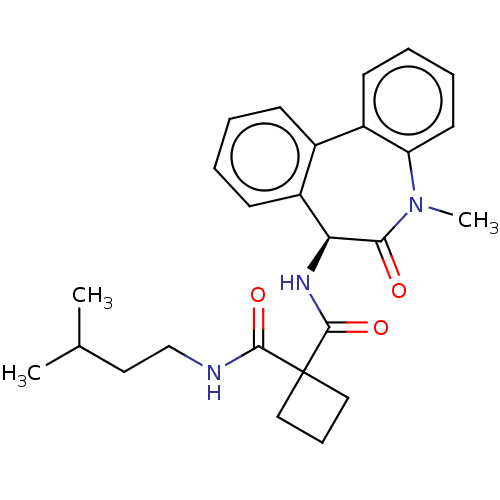
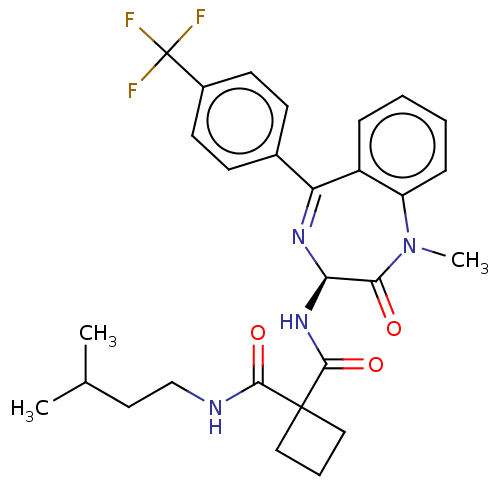
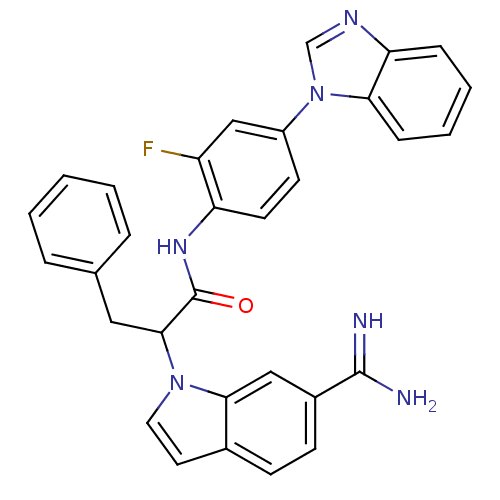
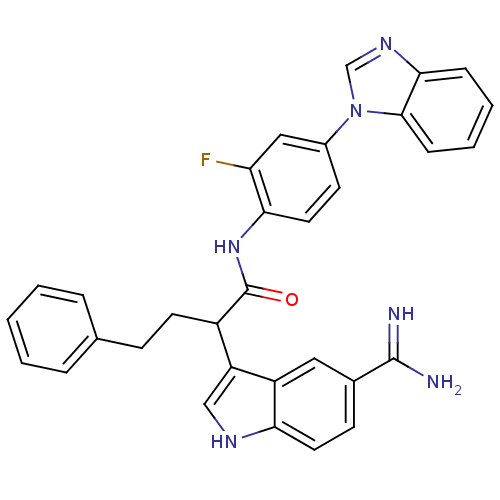
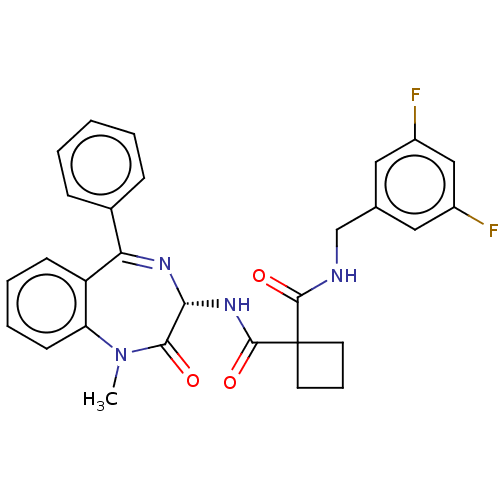
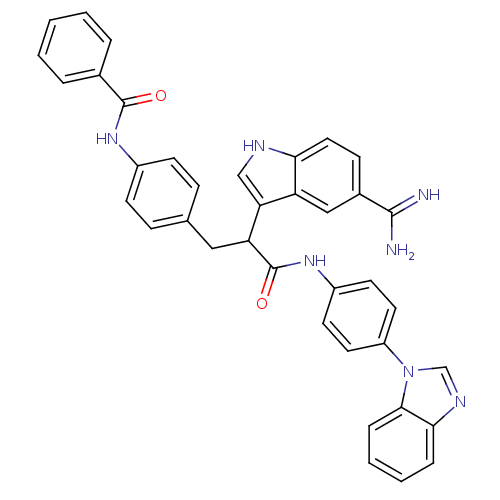
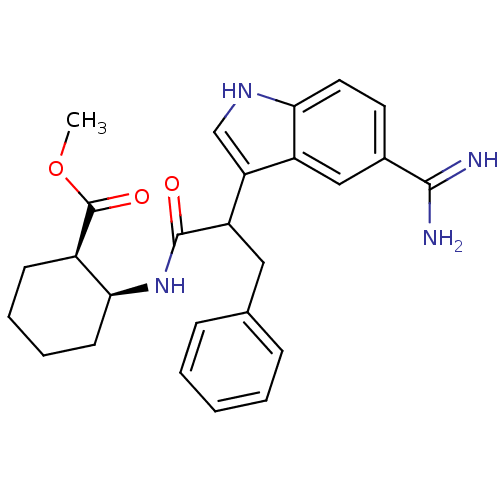
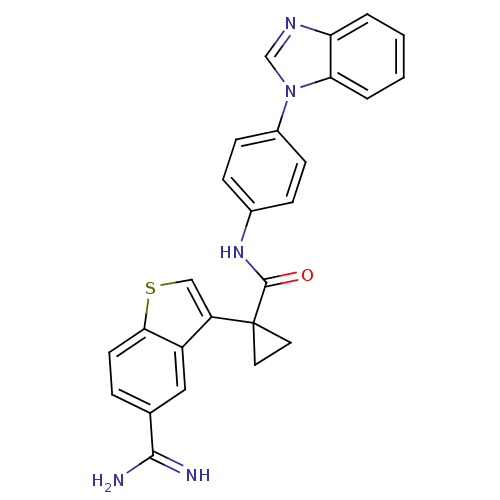
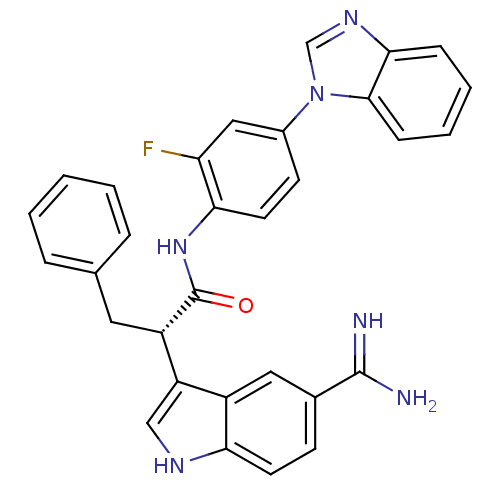
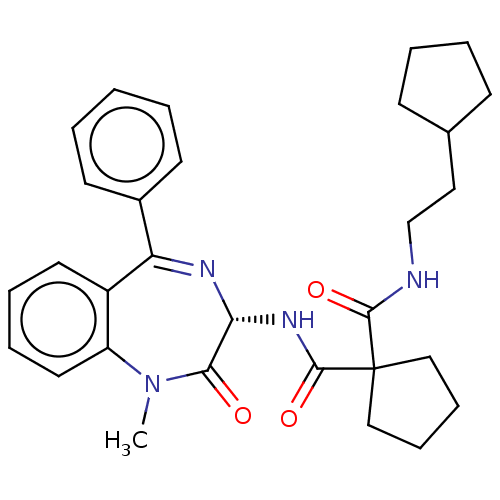
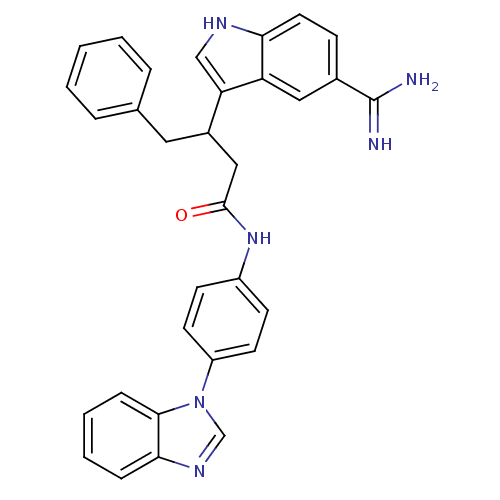
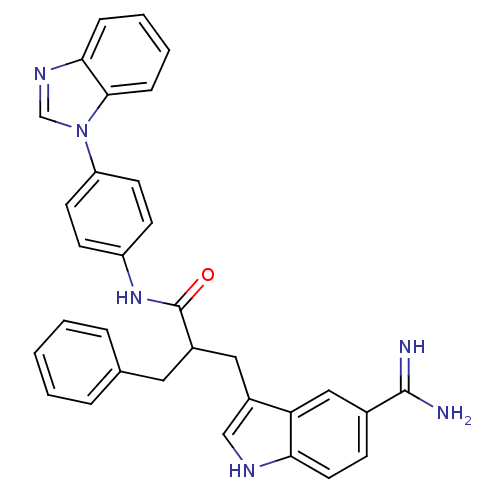
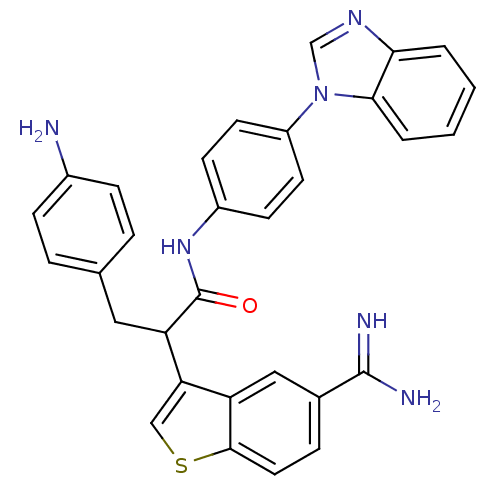
Affinity DataKi: 0.630nM ΔG°: -52.0kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nM ΔG°: -50.2kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H...More data for this Ligand-Target Pair
Affinity DataKi: 1.80nM ΔG°: -49.4kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.10nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -48.9kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -48.6kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nM ΔG°: -48.6kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 2.80nM ΔG°: -48.3kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.20nM ΔG°: -48.0kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 3.5nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -47.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 4.90nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 5.60nM ΔG°: -46.6kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 6.10nM ΔG°: -46.4kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7nM ΔG°: -46.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -44.4kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 18nM ΔG°: -43.8kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 19nM ΔG°: -43.6kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 22nM ΔG°: -43.3kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -42.9kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 28nM ΔG°: -42.7kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.1kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Displacement of [3H]succinamide from gamma secretase in THP1 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 45nM ΔG°: -41.5kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair
Affinity DataKi: 47nM ΔG°: -41.4kJ/molepH: 7.0 T: 2°CAssay Description:Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter...More data for this Ligand-Target Pair



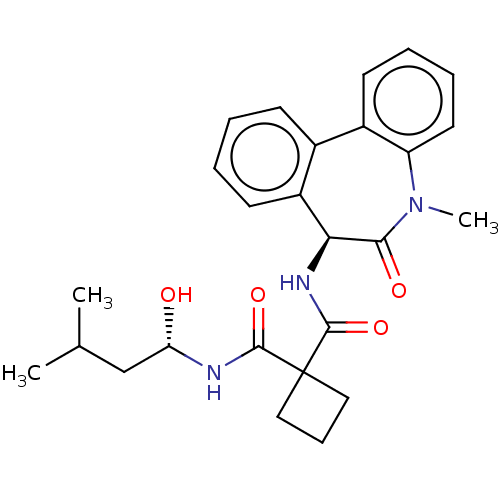

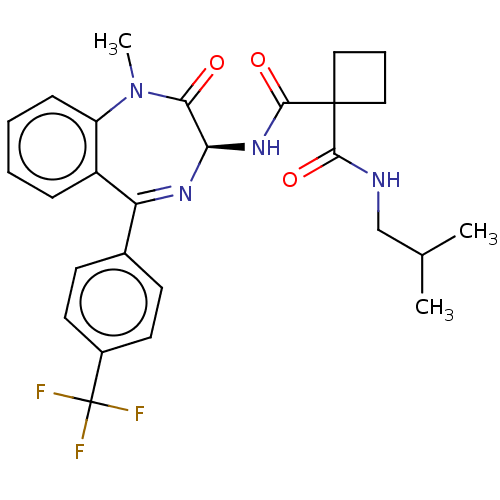
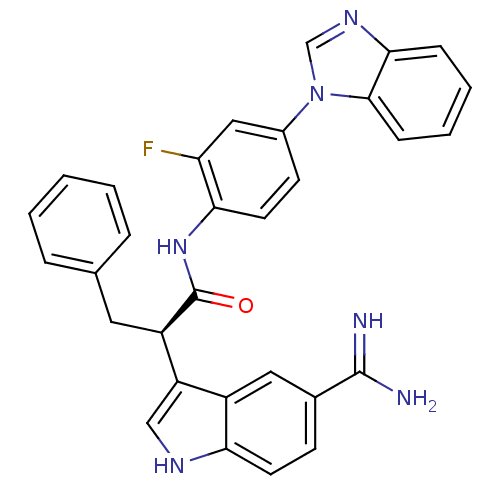

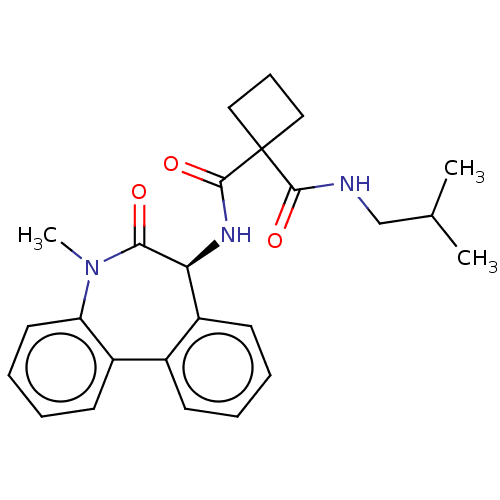
 3D Structure (crystal)
3D Structure (crystal)