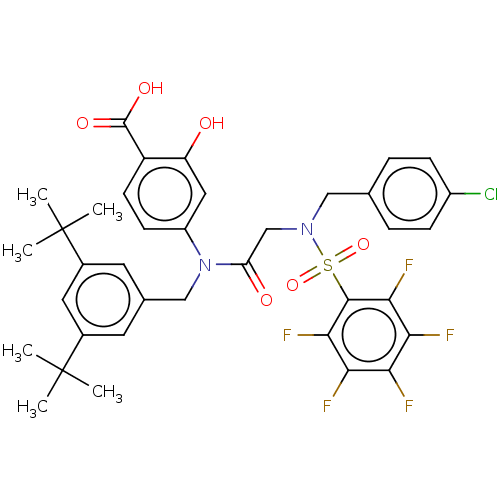
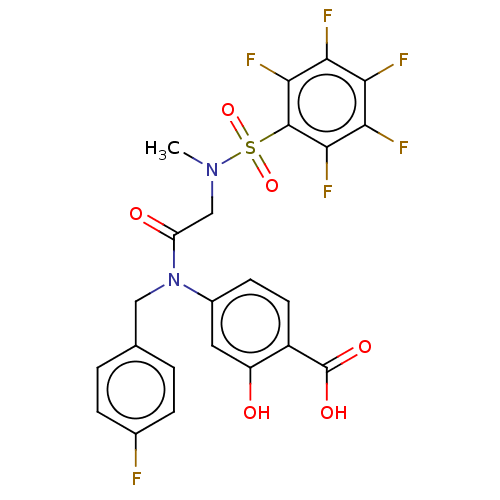
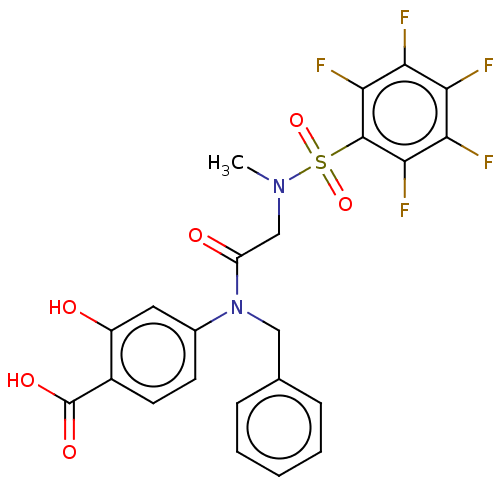
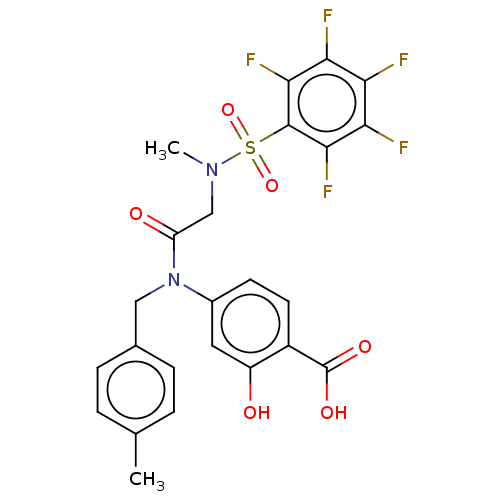
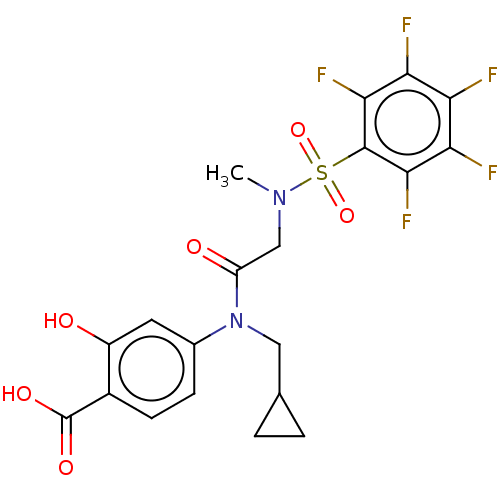
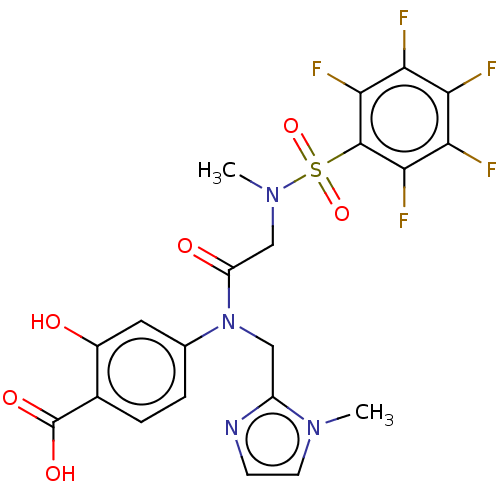
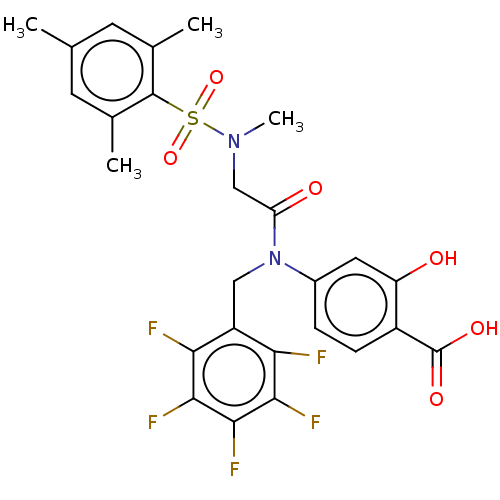
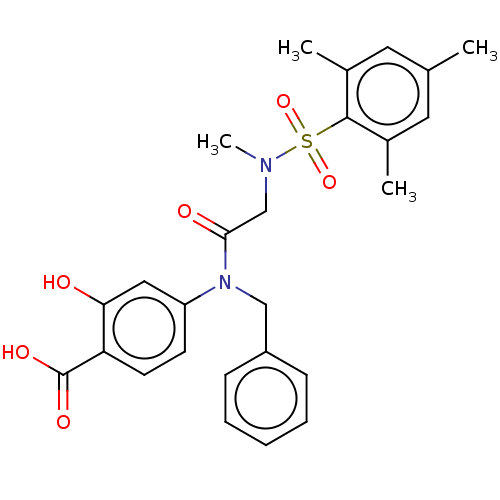
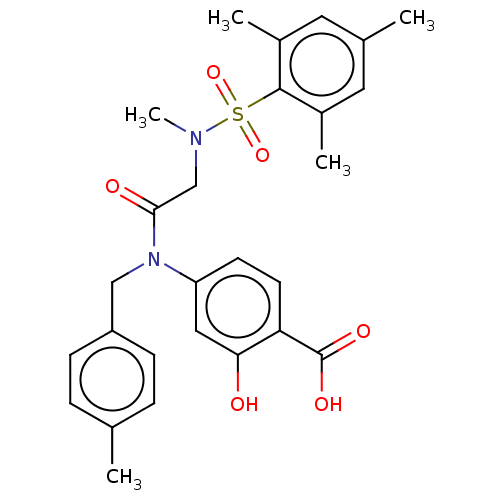
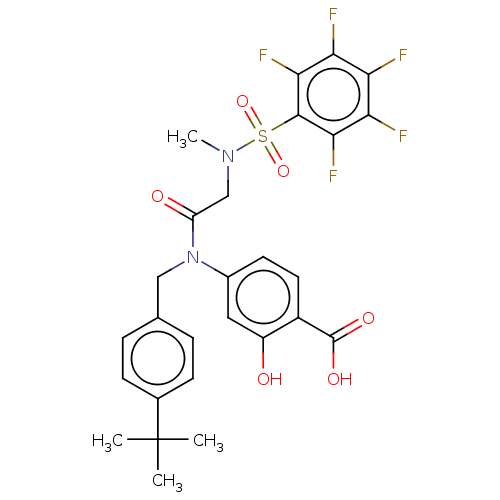
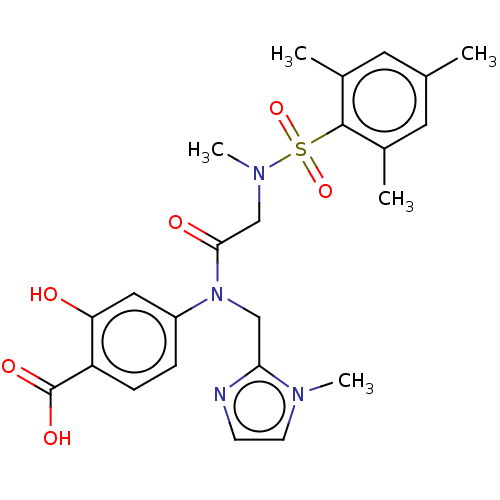
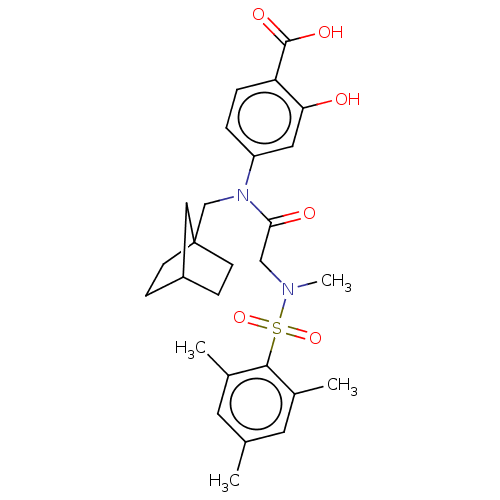
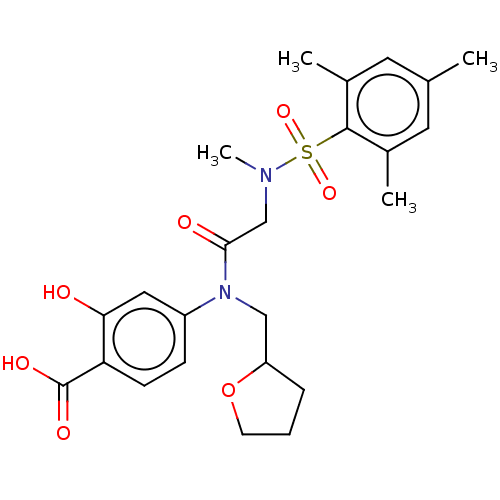
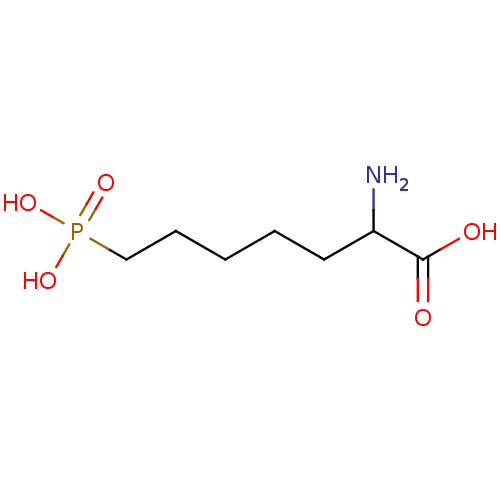
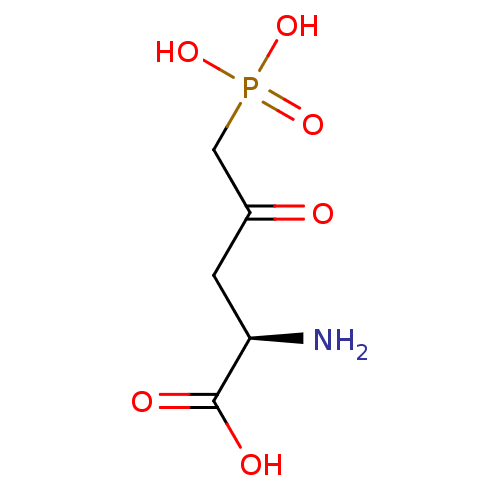
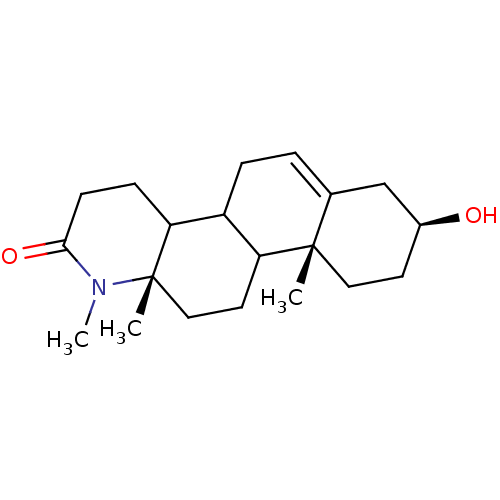
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 146nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
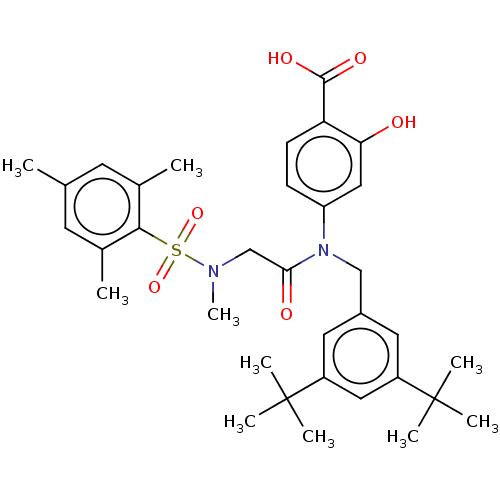
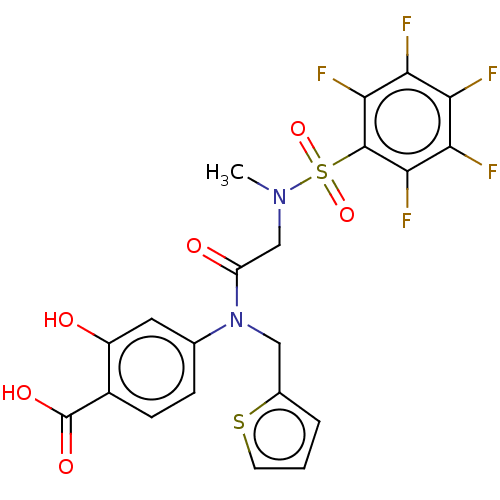
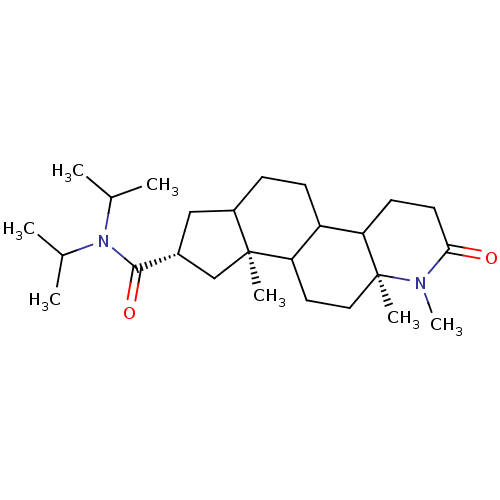
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 4.77E+3nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
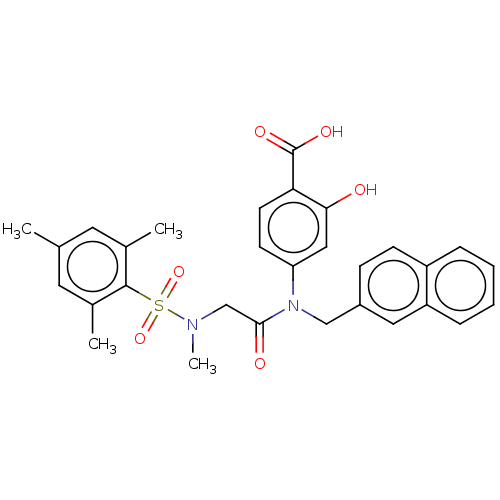
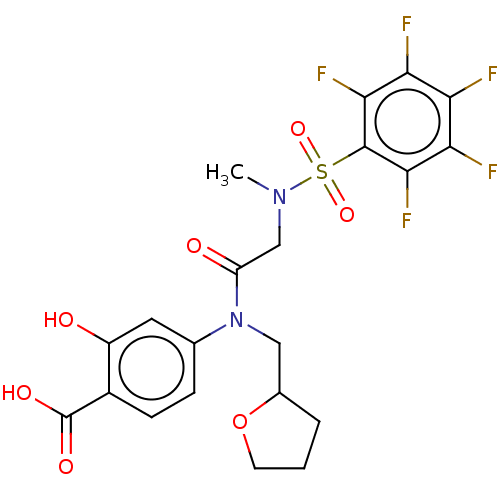
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 6.62E+3nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
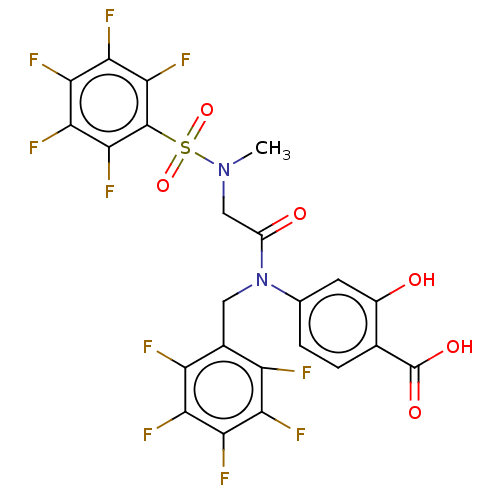
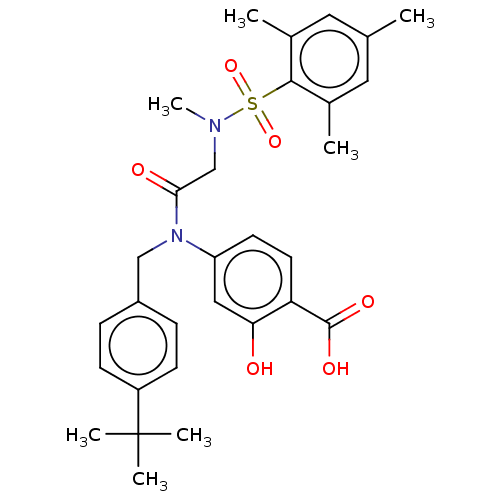
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 1.15E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 1.24E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: >6.00E+4nMAssay Description:Inhibition of STAT5B SH2 domain (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards AMPA receptor using [3H]AMPA as radioligand; InactiveMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 3(Rattus norvegicus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity towards Ionotropic glutamate receptor kainate using [3H]-kainic acid as radioligand; InactiveMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKi: 1.44E+5nMAssay Description:Inhibition of STAT3 (unknown origin)-5-FAM-GpYLVLDKW interaction compound treated for 15 mins by fluorescent polarization assayMore data for this Ligand-Target Pair
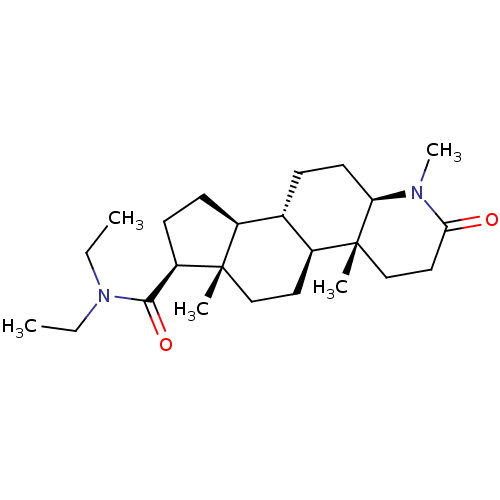
Affinity DataIC50: 8.60nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
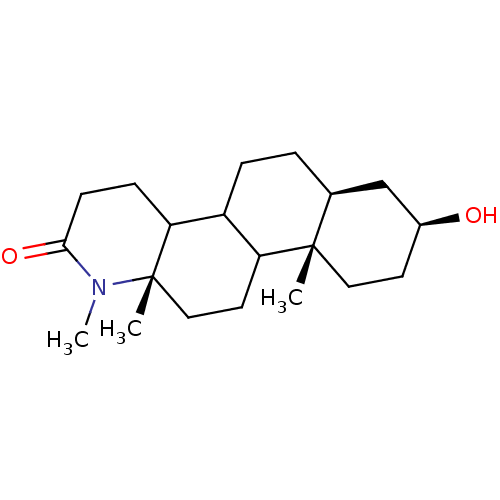
Affinity DataIC50: 430nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
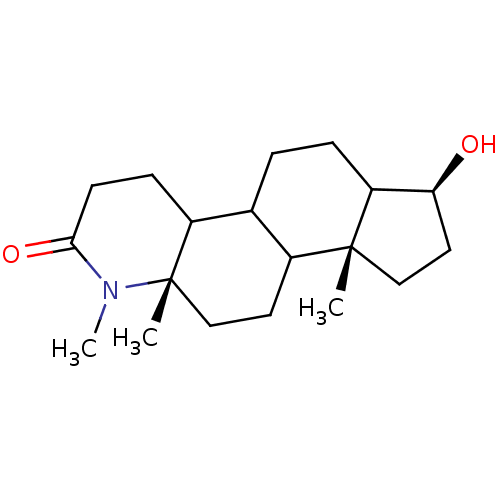
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity of the compound against human prostatic Steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKd: 287nMAssay Description:Binding affinity to full-length His-tagged STAT3 (unknown origin) by surface plasmon resonance assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 5B(Homo sapiens (Human))
University of Toronto Mississauga
Curated by ChEMBL
University of Toronto Mississauga
Curated by ChEMBL
Affinity DataKd: 42nMAssay Description:Binding affinity to full-length His-tagged STAT5B (unknown origin) by surface plasmon resonance assayMore data for this Ligand-Target Pair