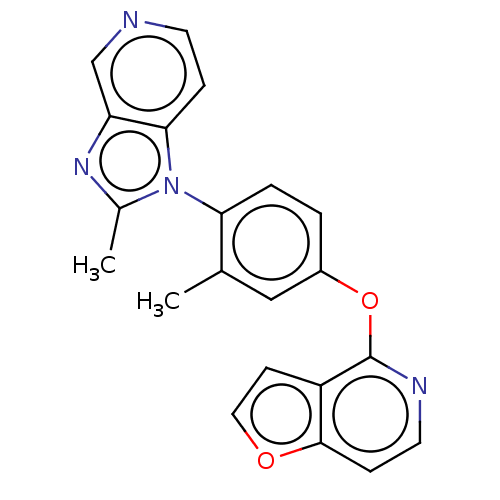
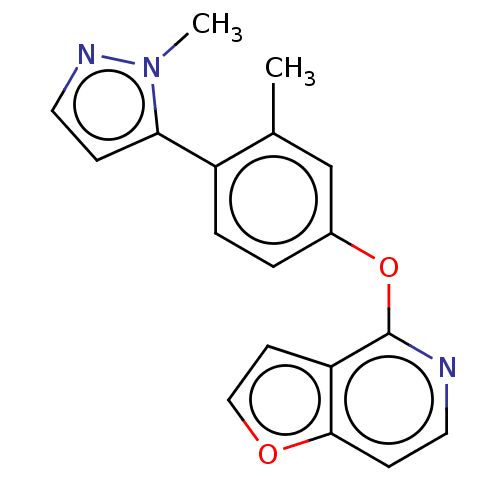
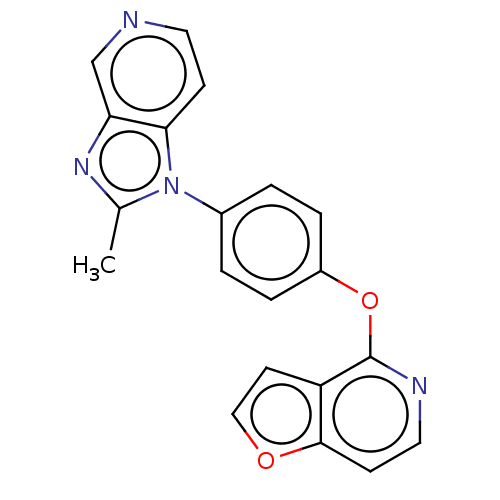
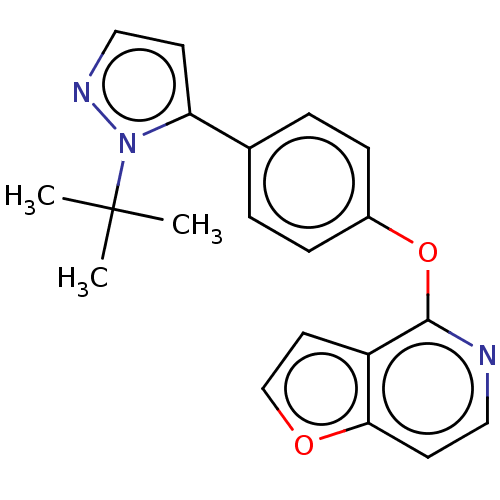
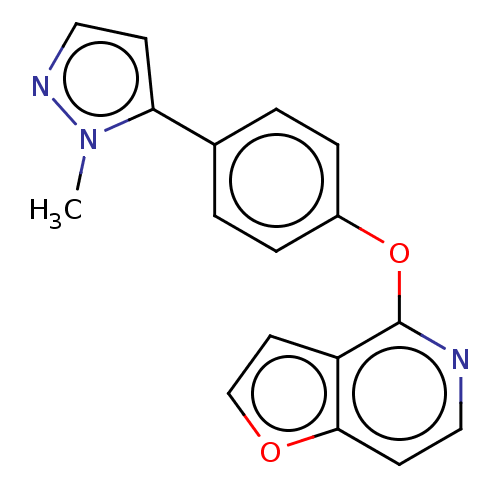
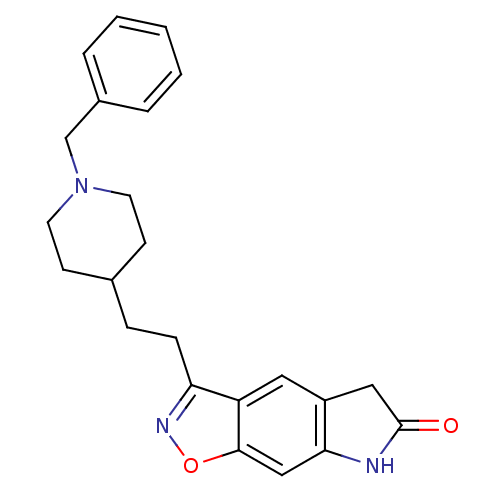
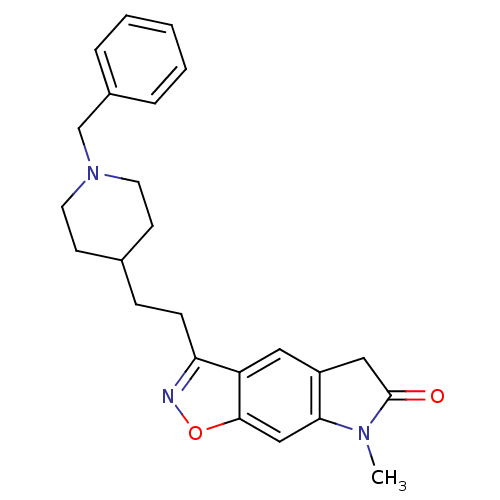
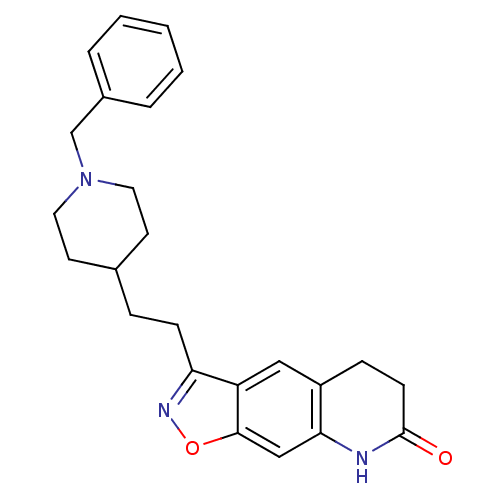
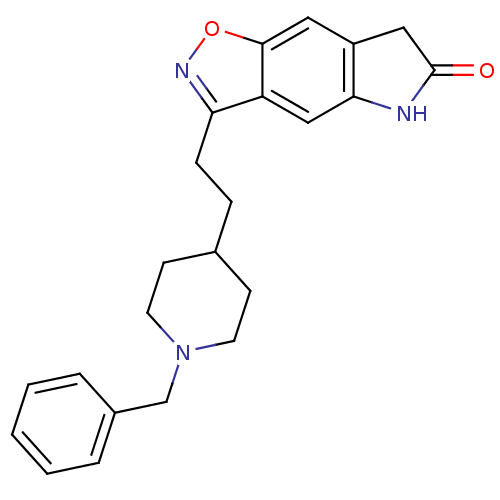
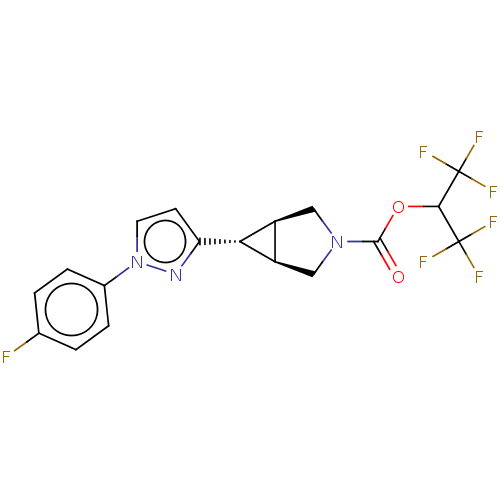
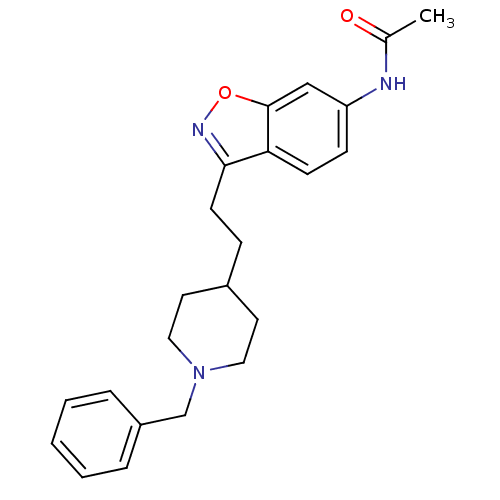
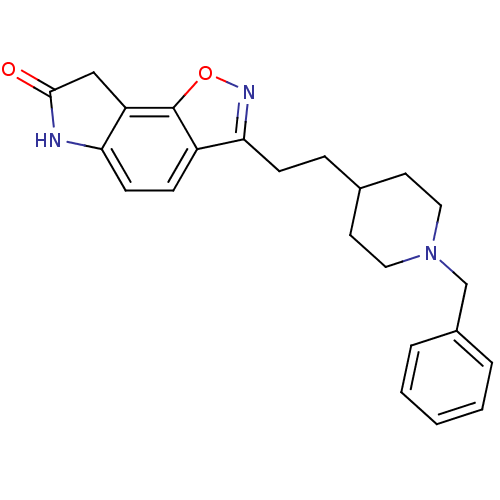
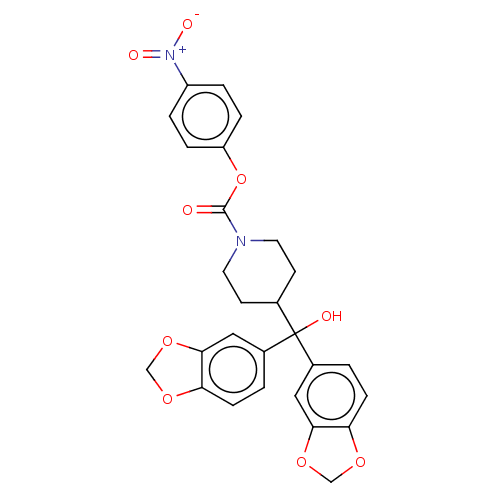
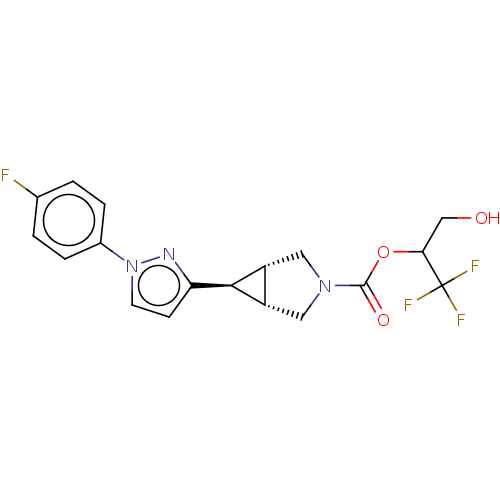
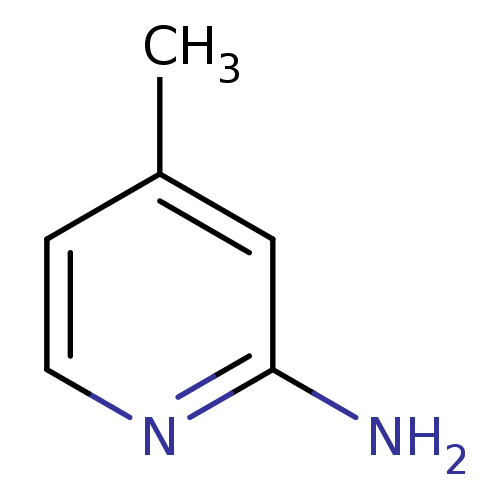
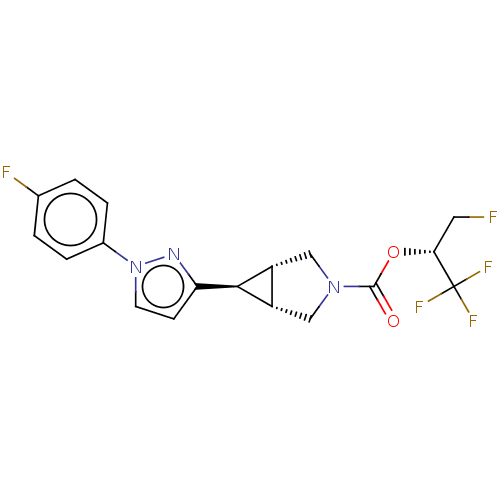
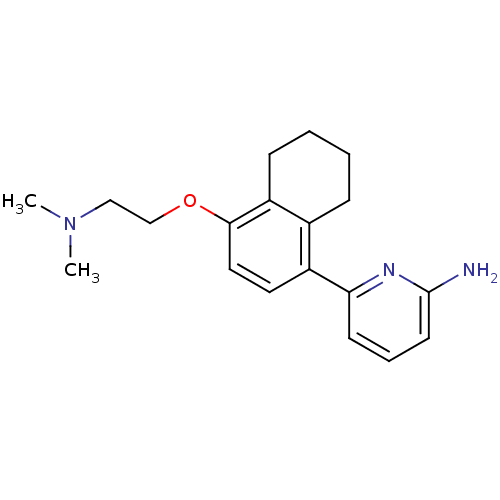
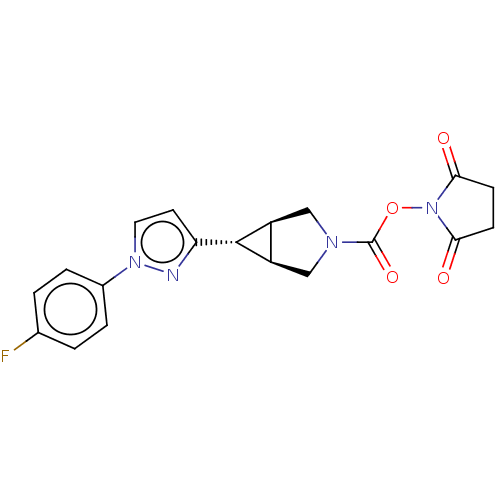
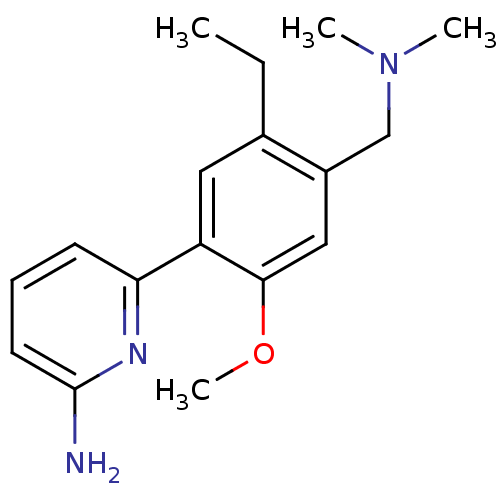
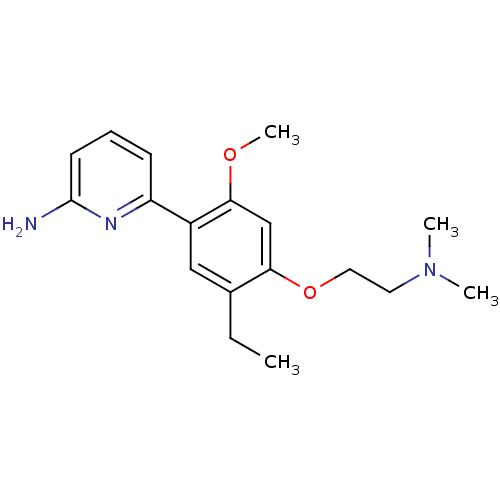
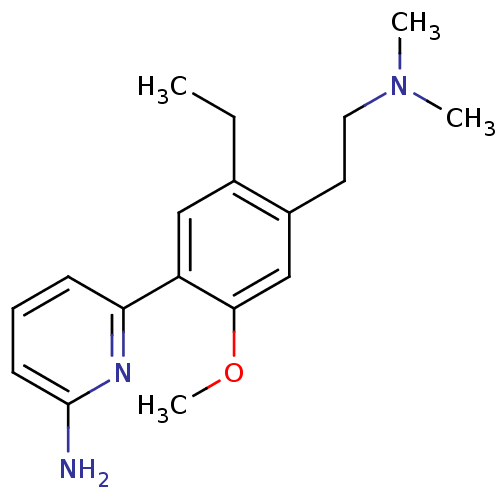
Affinity DataKi: 4.80nMAssay Description:Agonist activity at dopamine D5 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.5nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 21.6nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 99nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 121nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 243nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 244nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 254nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 376nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 563nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.68E+3nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.24E+3nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.35E+3nMAssay Description:Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Agonist activity at dopamine D2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.480nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of Butyrylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:In vitro inhibition of human inducible nitric oxide synthaseMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of recombinant human FAAH using fluorogenic arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:In vitro inhibition of Acetylcholinesterase from human erythrocytesMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 21.8nMAssay Description:In vitro inhibition of rat neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:In vitro inhibition of rat neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of rat neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:In vitro inhibition of human neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetMonoglyceride lipase(Homo sapiens (Human))
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States.
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:In vitro inhibition of rat neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 50.6nMAssay Description:In vitro inhibition of human neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibitory activity against neuronal nitric oxide synthase in humanMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 58nMAssay Description:Inhibitory activity against neuronal nitric oxide synthase in humanMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 59nMAssay Description:In vitro inhibition of rat neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 70.3nMAssay Description:In vitro inhibition of human neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 70.9nMAssay Description:In vitro inhibition of human neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:In vitro inhibition of Butyrylcholinesterase from human serumMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibitory activity against neuronal nitric oxide synthase in humanMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibitory activity against neuronal nitric oxide synthase in humanMore data for this Ligand-Target Pair




 3D Structure (crystal)
3D Structure (crystal)