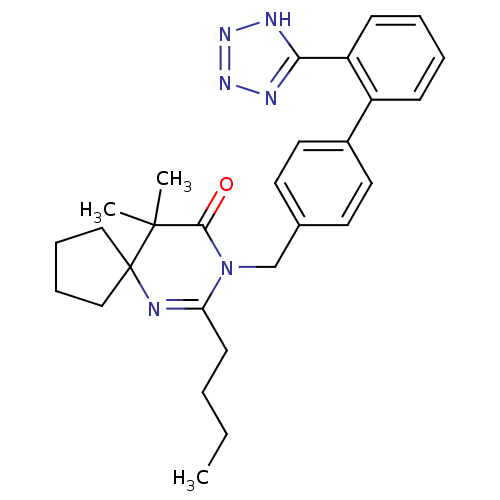
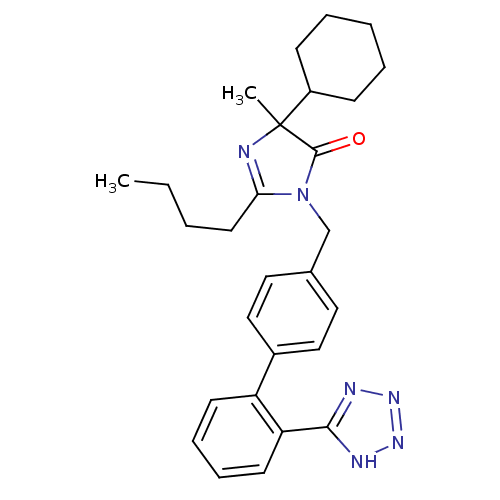
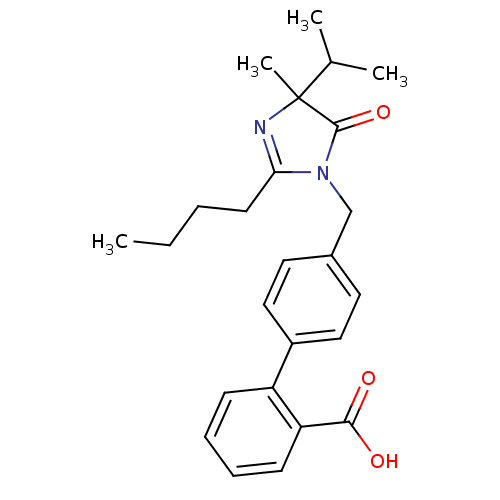
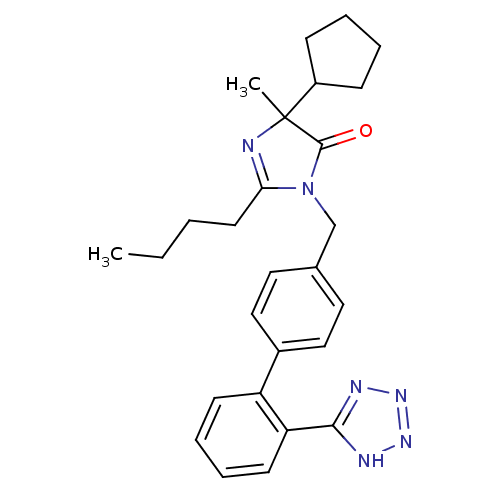
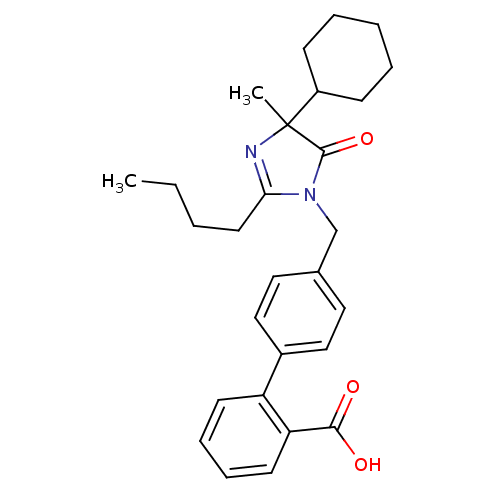
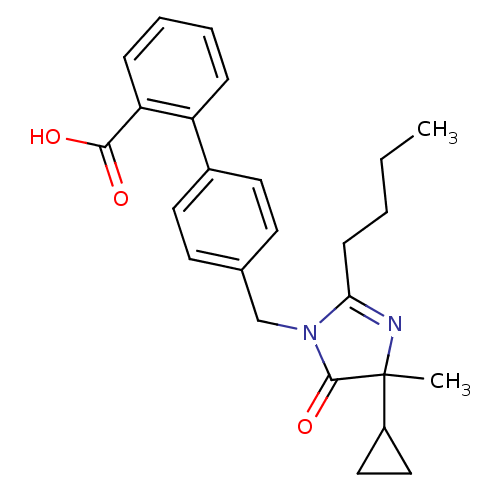
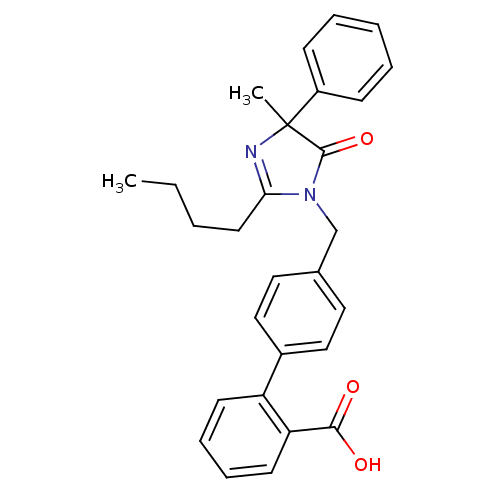
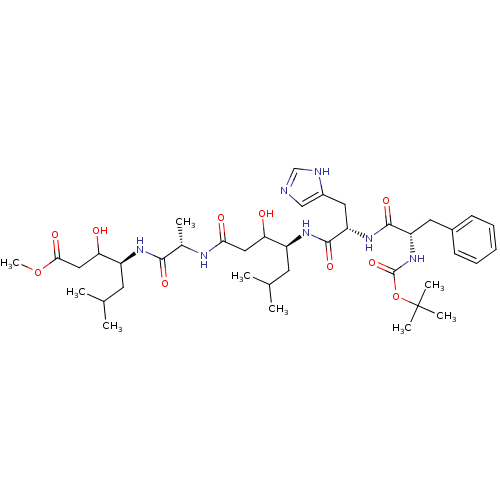
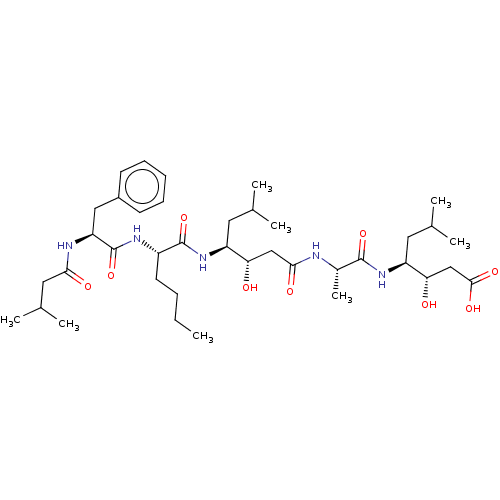
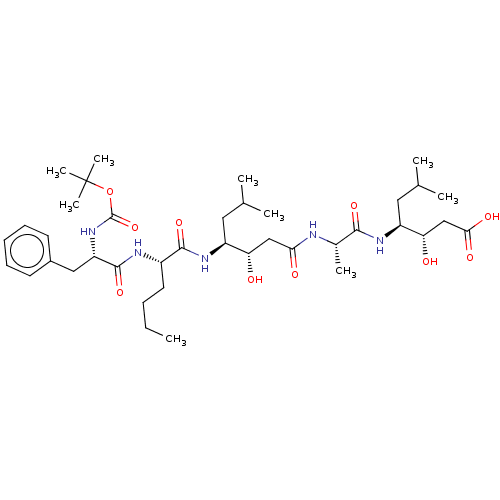
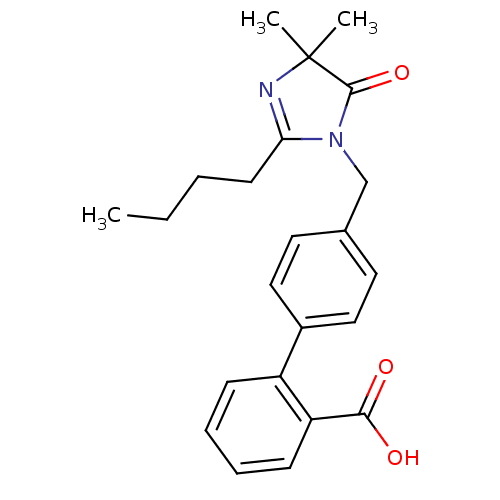
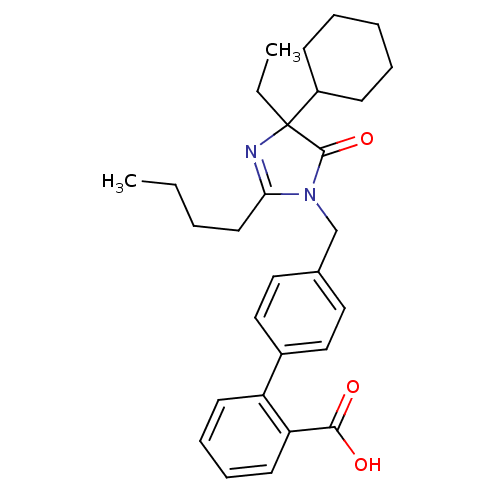
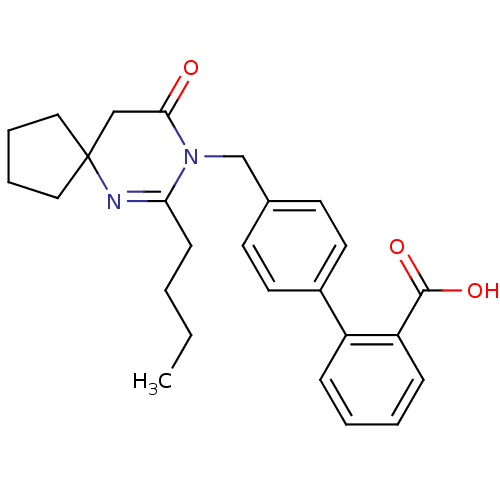
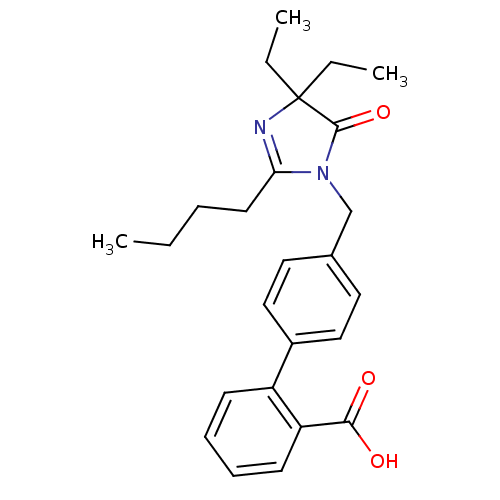
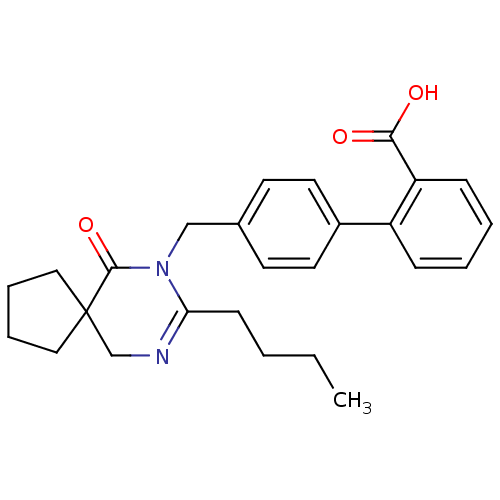
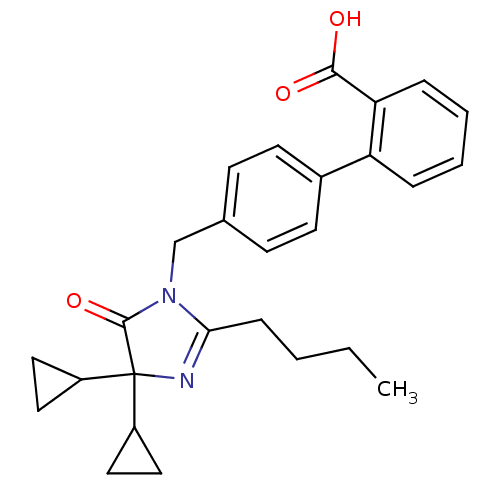
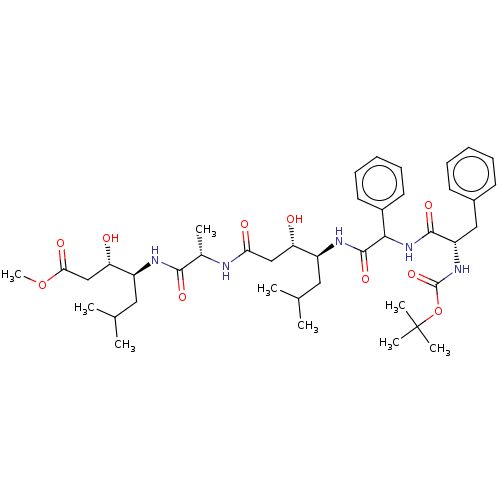
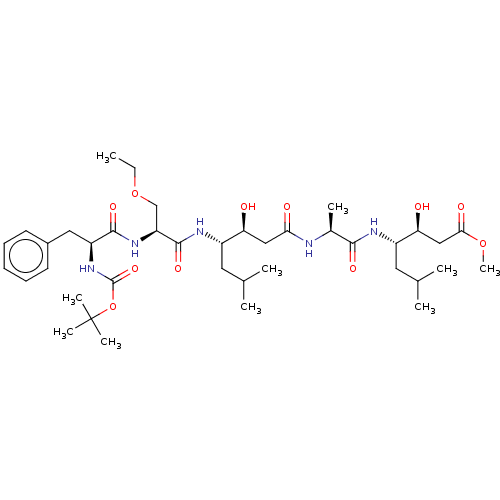
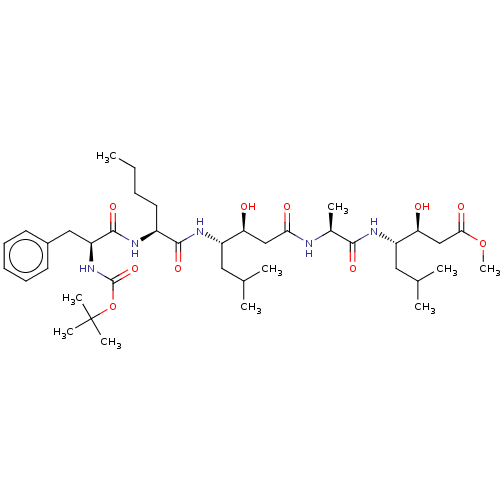
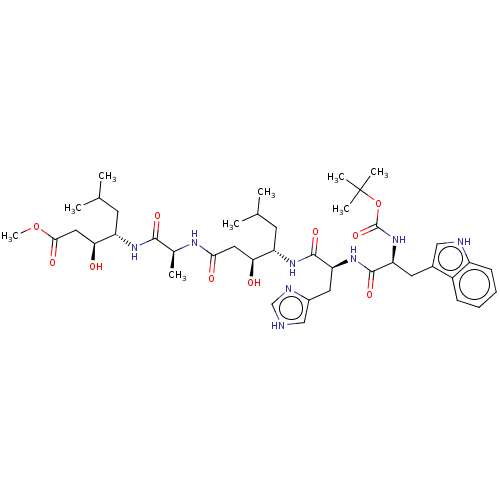
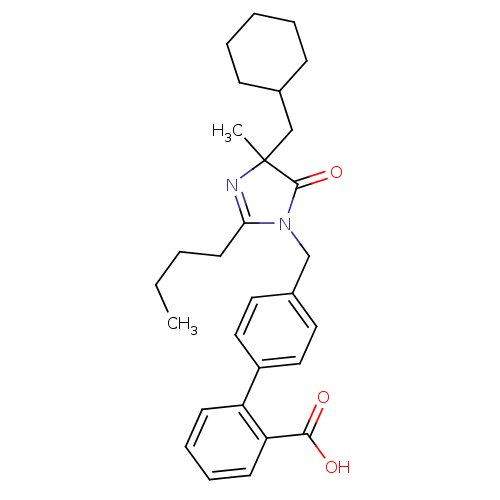
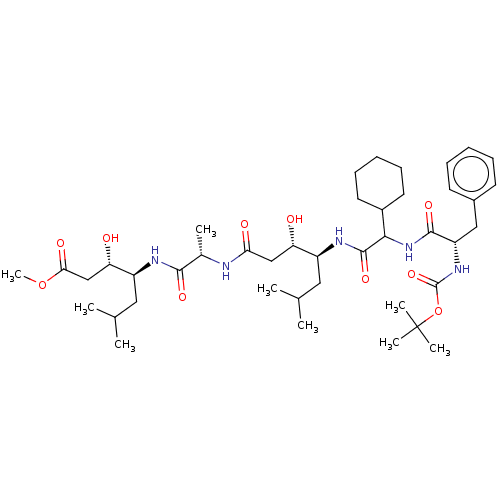
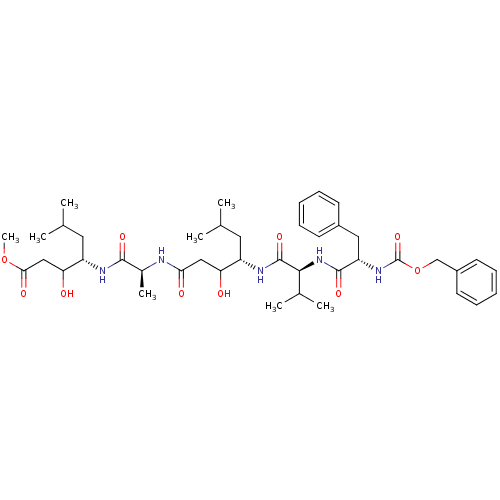
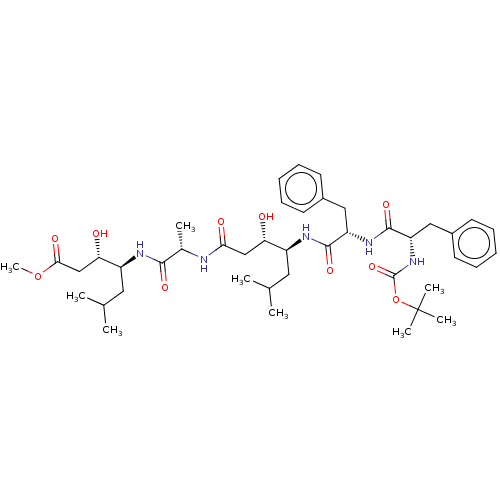
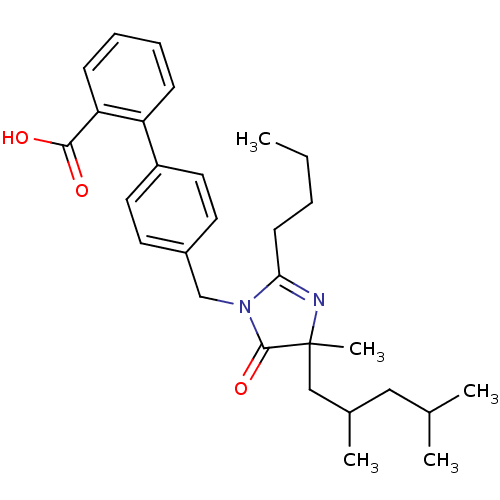
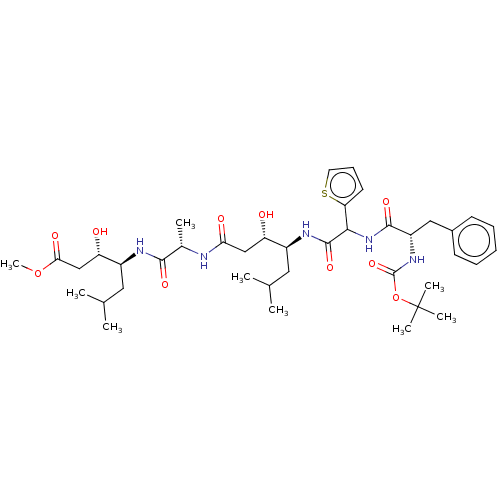
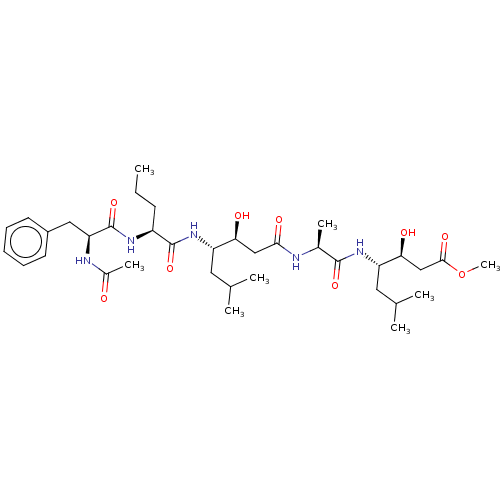
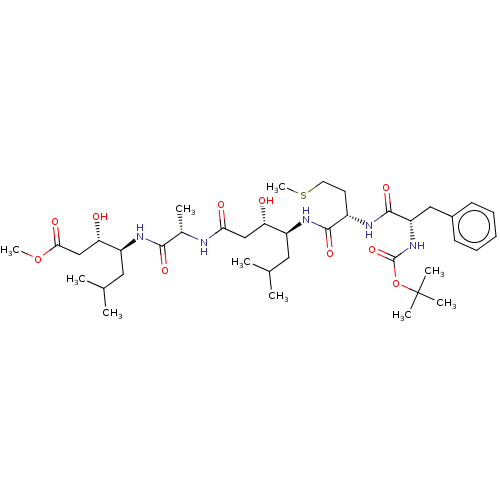
TargetVasopressin V1b receptor(Homo sapiens (Human))
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
TargetVasopressin V1b receptor(Homo sapiens (Human))
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
TargetVasopressin V1a receptor(Homo sapiens (Human))
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
TargetVasopressin V2 receptor(Homo sapiens (Human))
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
TargetVasopressin V2 receptor(Rattus norvegicus (Rat))
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
Affinity DataIC50: 1nMAssay Description:In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 141nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 174nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 178nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 191nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement.More data for this Ligand-Target Pair
Affinity DataIC50: 251nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 257nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 316nMAssay Description:Inhibition of human plasma reninMore data for this Ligand-Target Pair