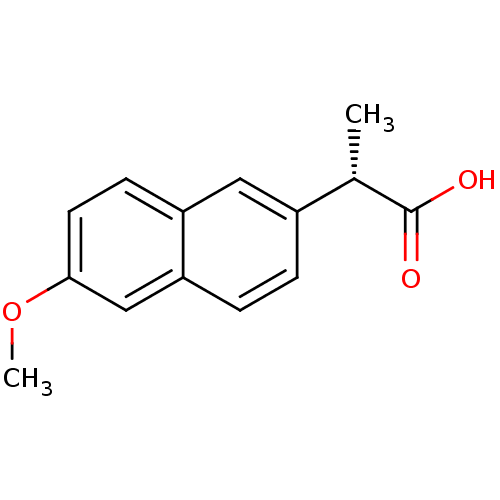
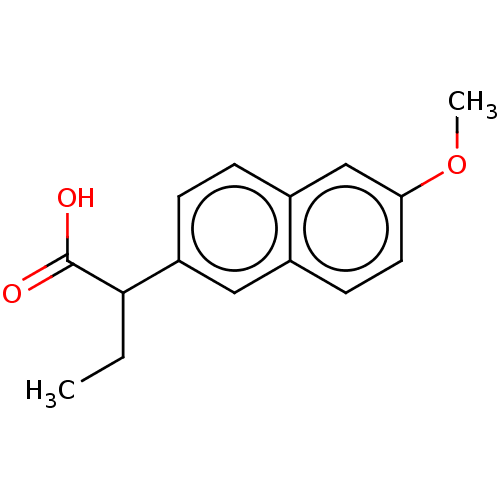
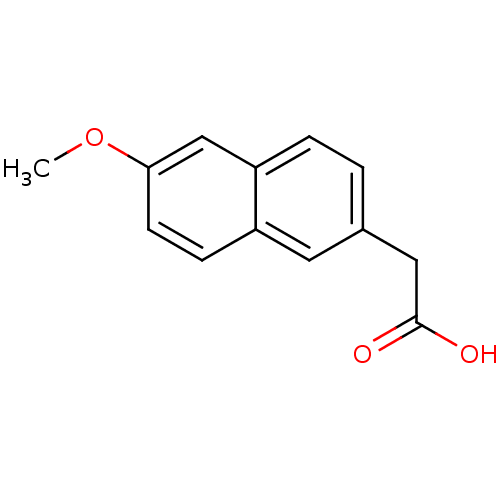
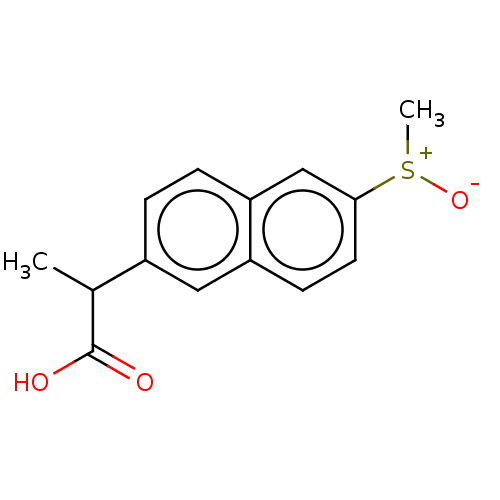
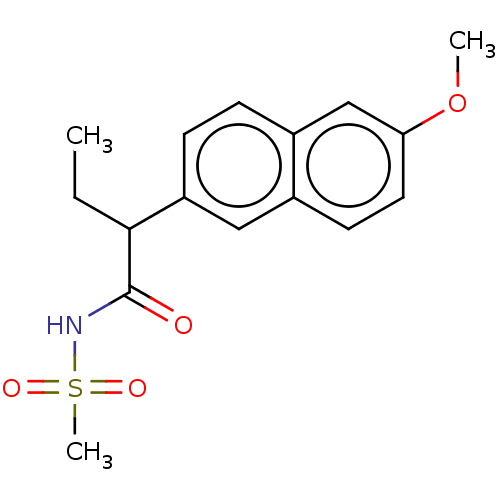
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Competitive inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preinc...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataKi: 750nMAssay Description:Competitive inhibition of human recombinant AKR1C3 using assessed as reduction in NADPH-dependent reduction of delat4-androsten-3,17-dione preincubat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 650nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 820nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxidation ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.05E+3nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.35E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.72E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.75E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 4.35E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of COX2 (unknown origin) using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxidation ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 4.81E+4nMAssay Description:Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
Curated by ChEMBL
University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human recombinant AKR1C1 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi...More data for this Ligand-Target Pair

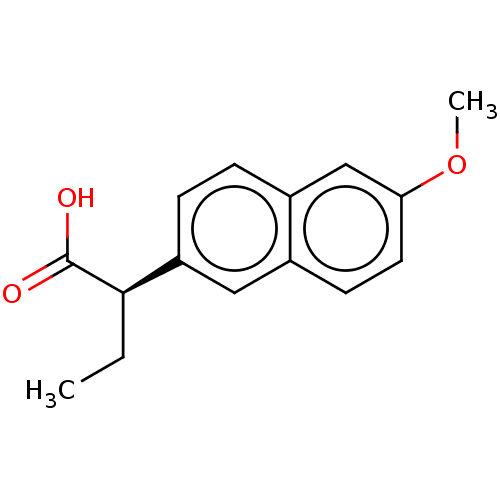
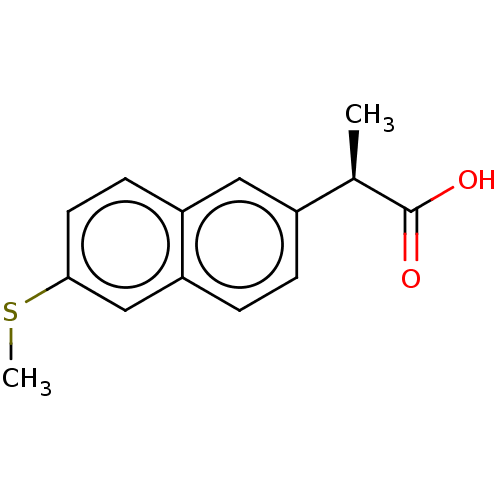
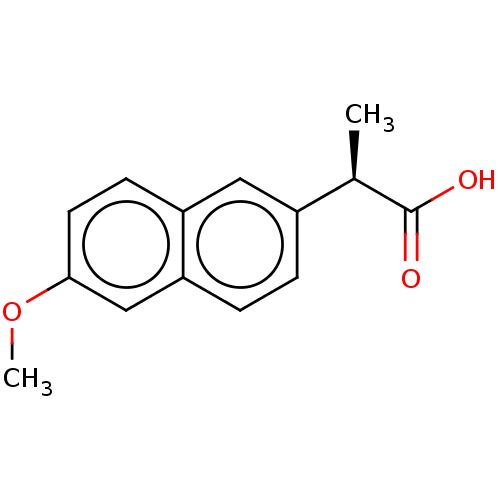
 3D Structure (crystal)
3D Structure (crystal)