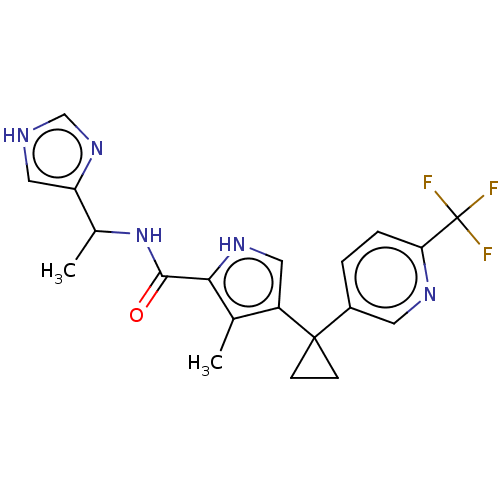
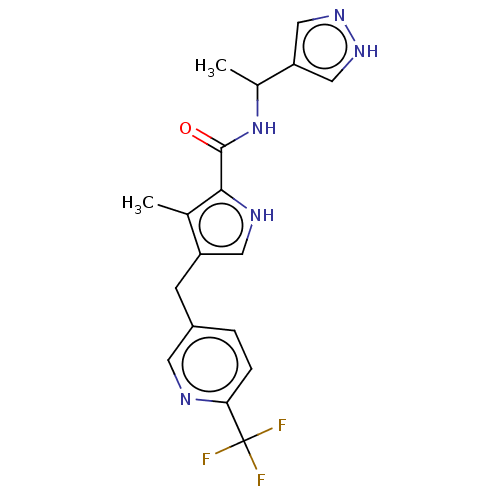
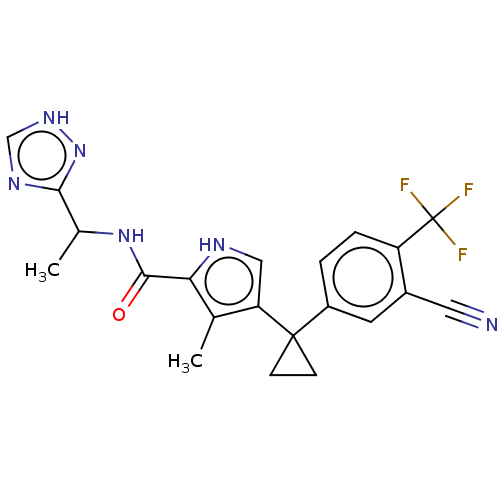
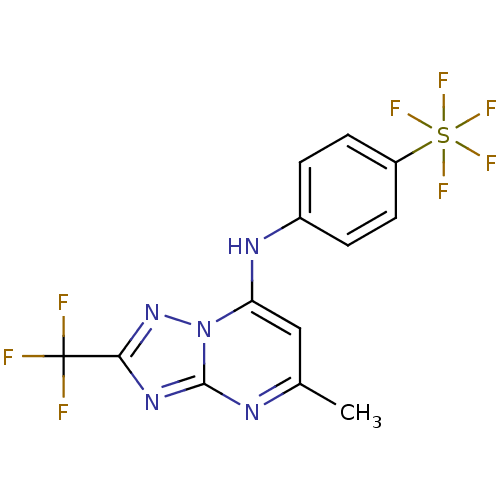
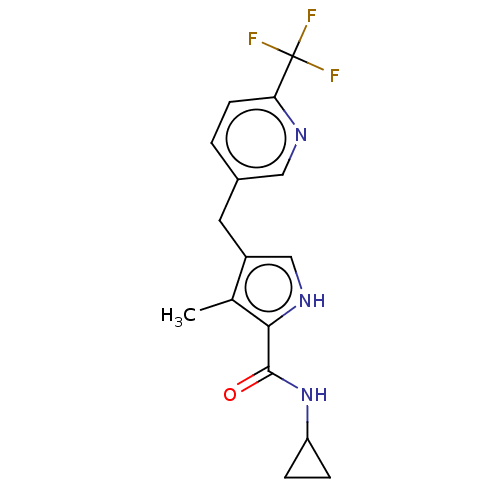
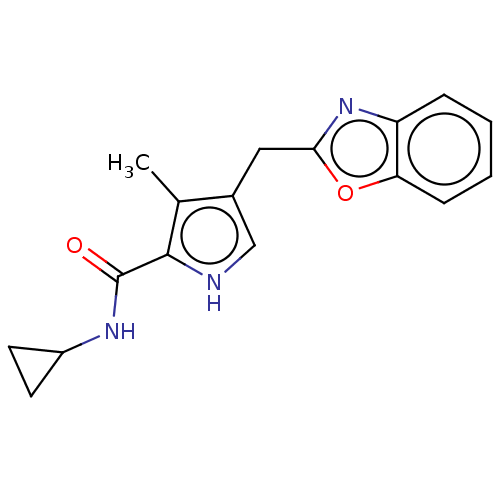
Affinity DataIC50: 250nMAssay Description:Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of NK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin) using phenacetin O-deethylation by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) by UPLC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+4nMAssay Description:Inhibition of human ERG by QPatch assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli BL21-DE3 using L-dihydroorotate as substrate by steady-state DCIP methodMore data for this Ligand-Target Pair