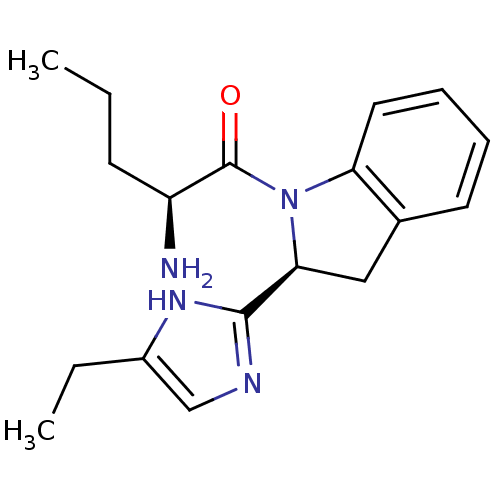
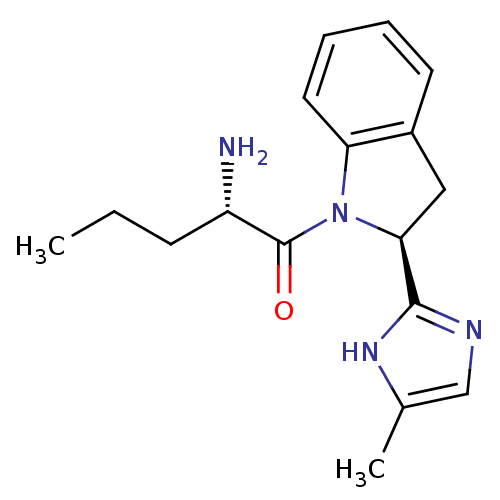
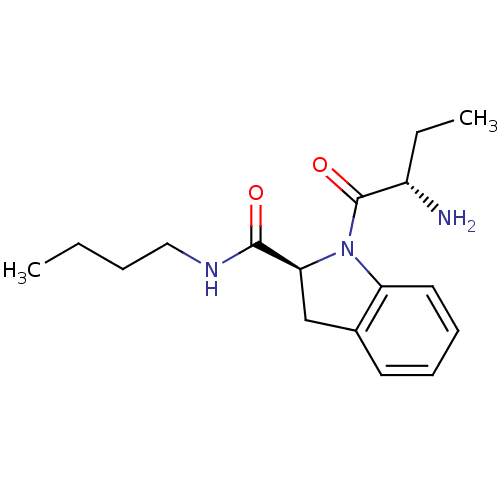
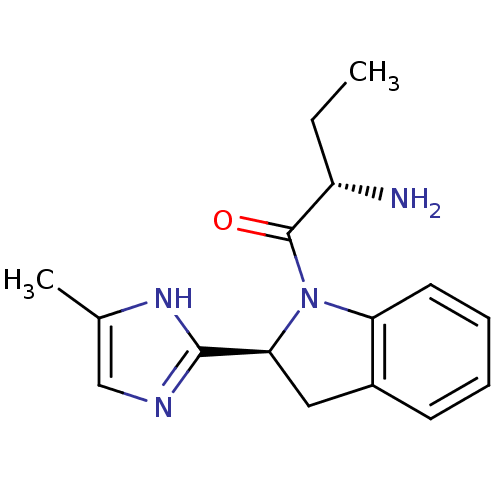
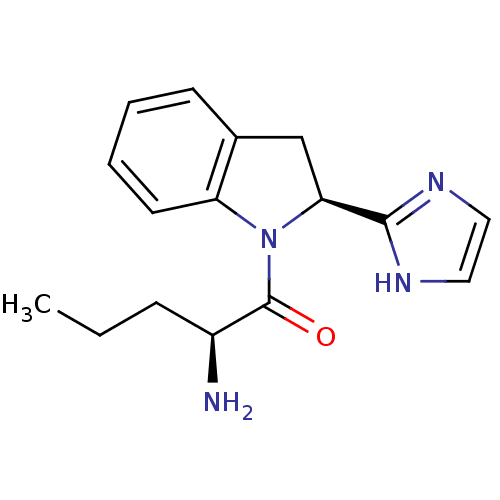
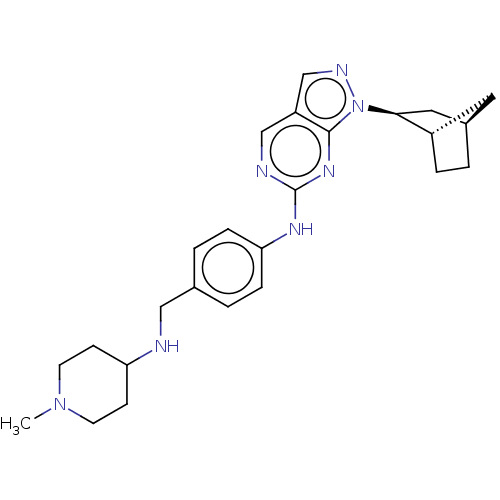
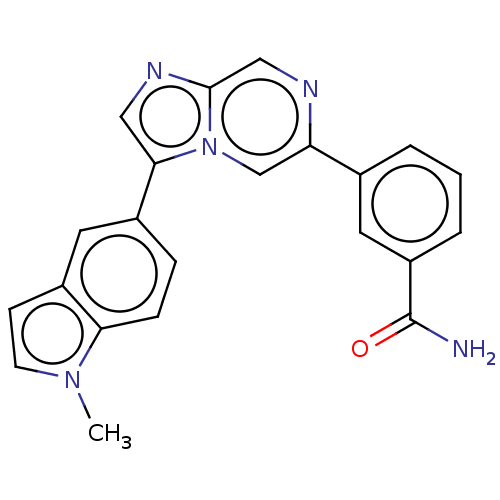
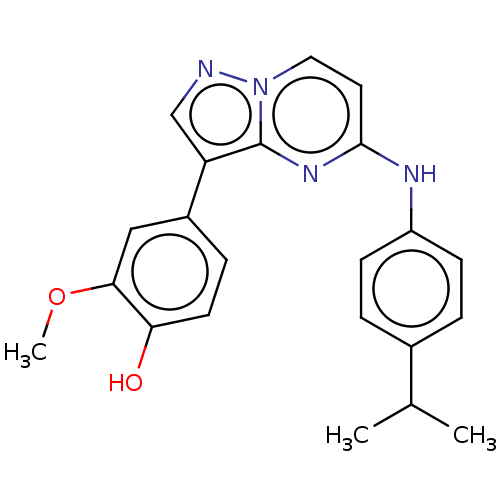
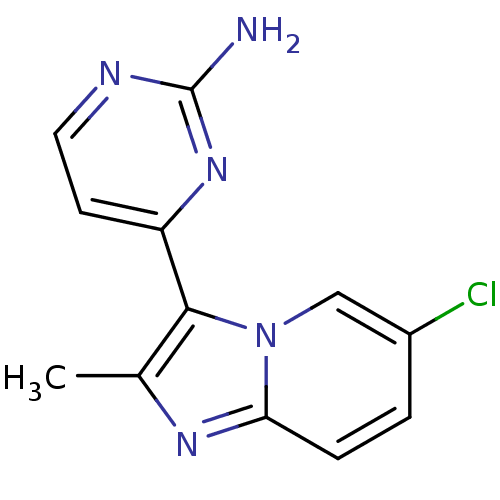
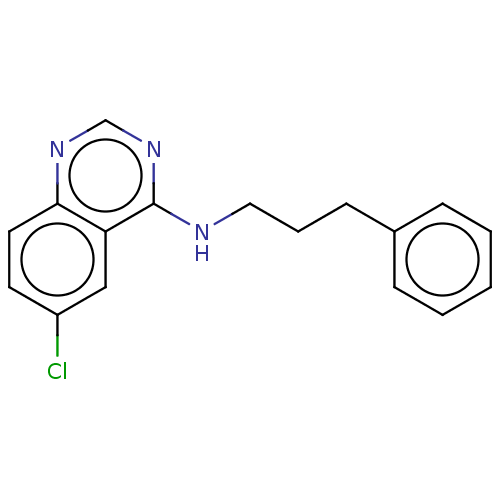
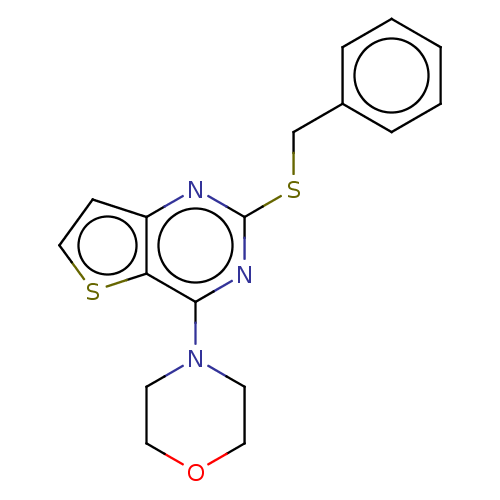
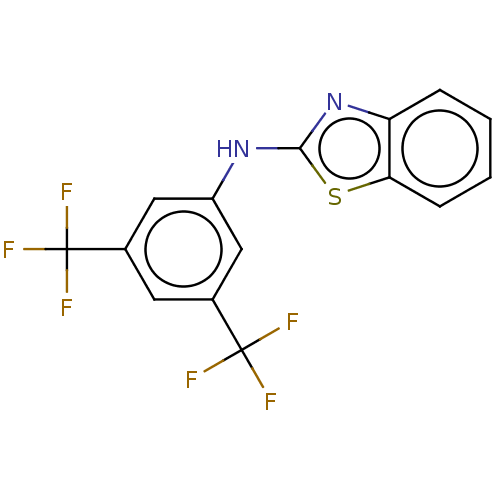
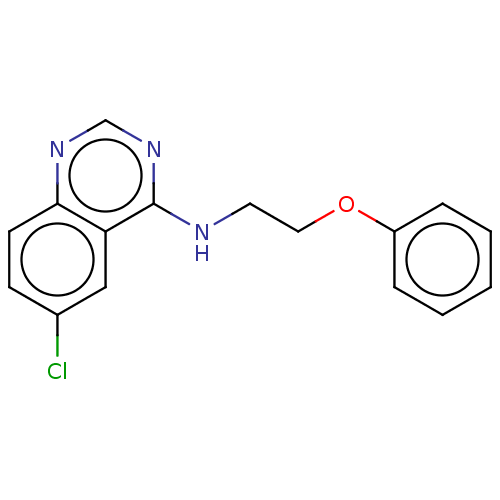
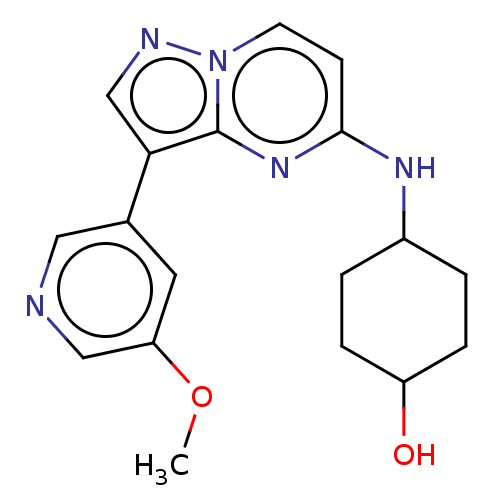
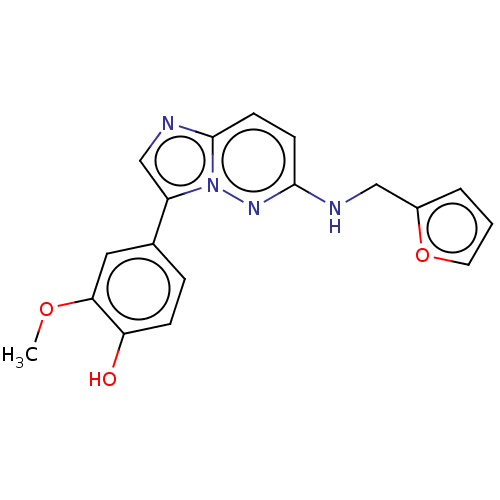
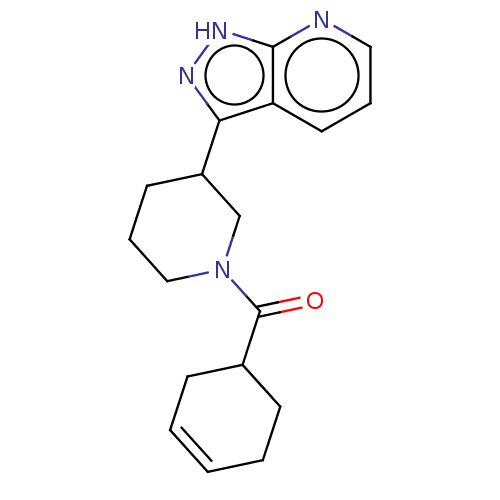
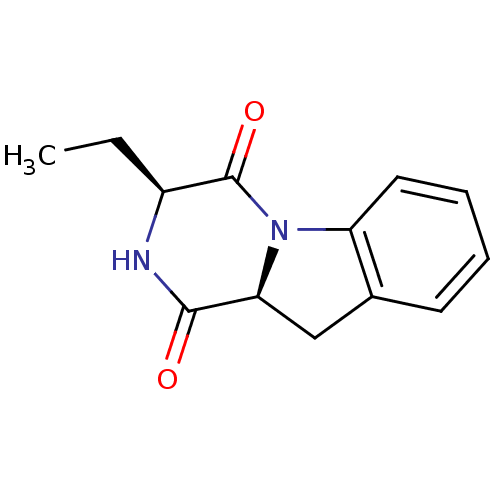
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 339nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 427nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 2.46E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 2.57E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 3.16E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 5.75E+3nMAssay Description:Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 6.17E+3nMAssay Description:Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 6.46E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 6.92E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 6.92E+3nMAssay Description:Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 8.13E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Janssen Research& Development
Curated by ChEMBL
Janssen Research& Development
Curated by ChEMBL
Affinity DataIC50: 8.51E+3nMAssay Description:Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind...More data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair
TargetTripeptidyl-peptidase 2(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liverMore data for this Ligand-Target Pair