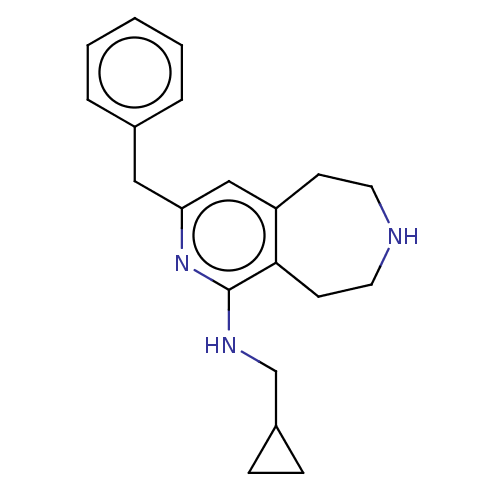
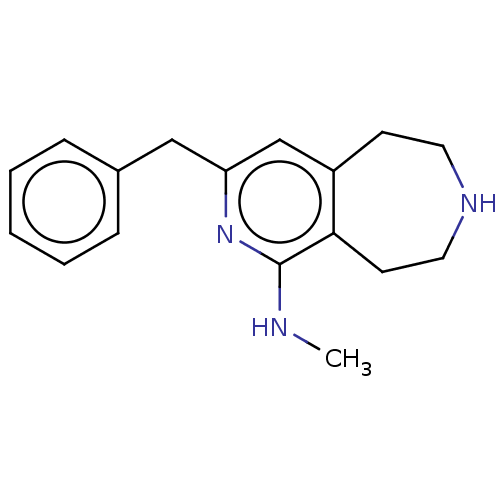
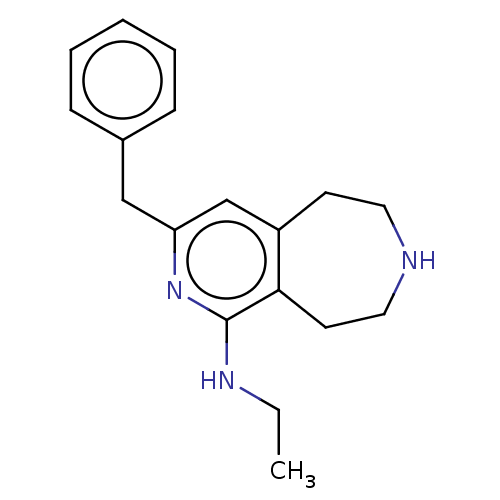
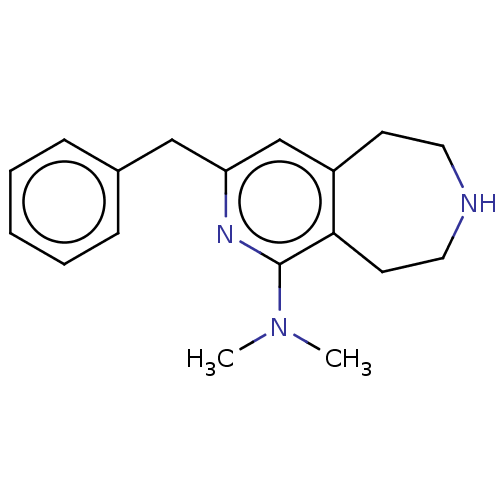
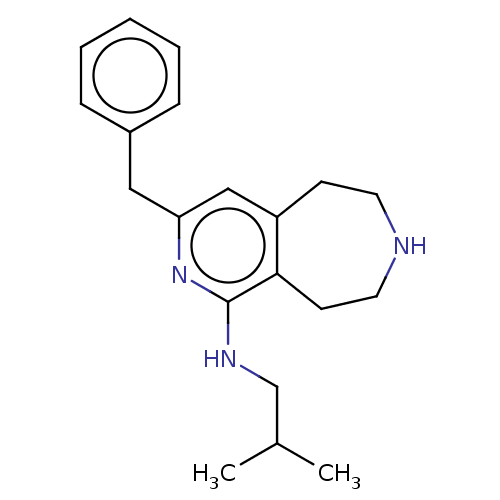
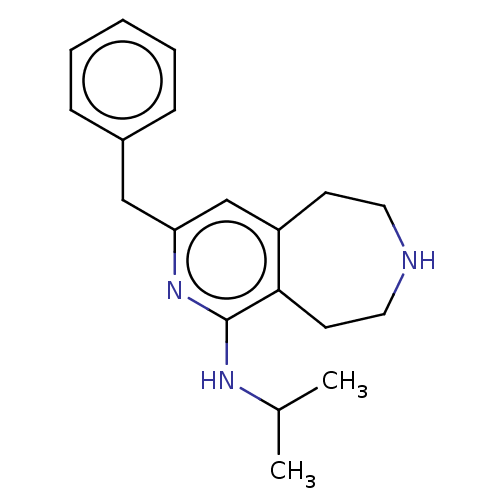
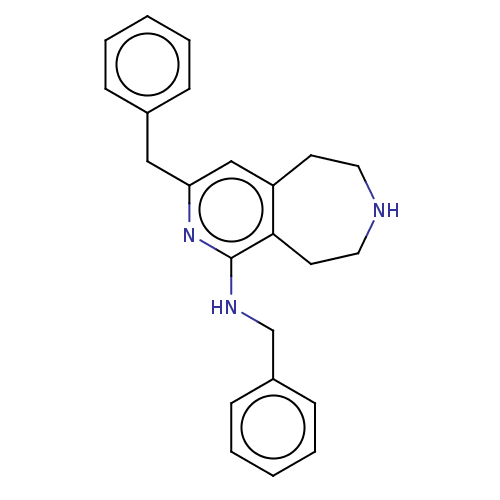
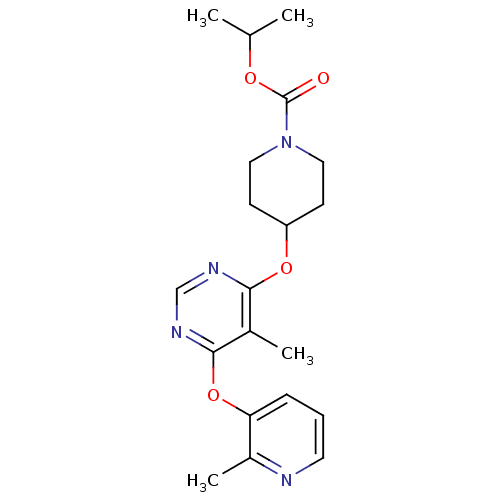
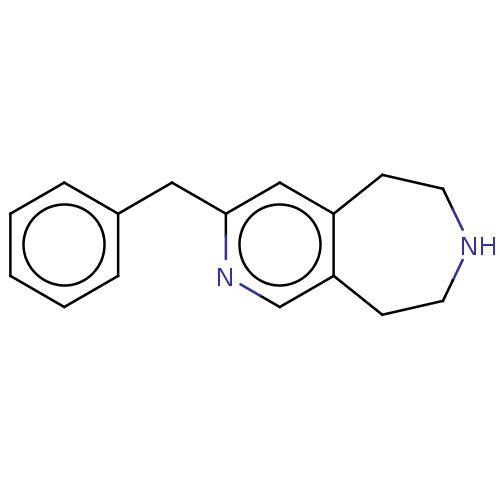
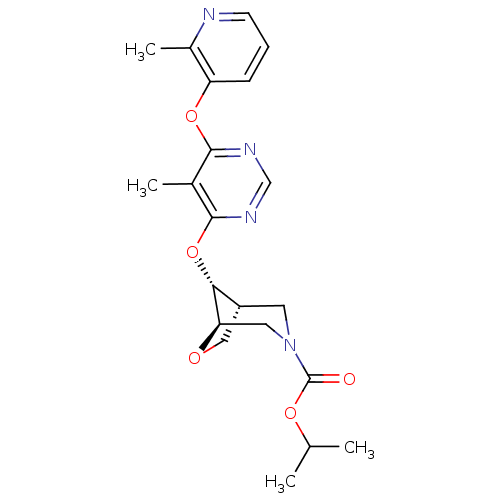
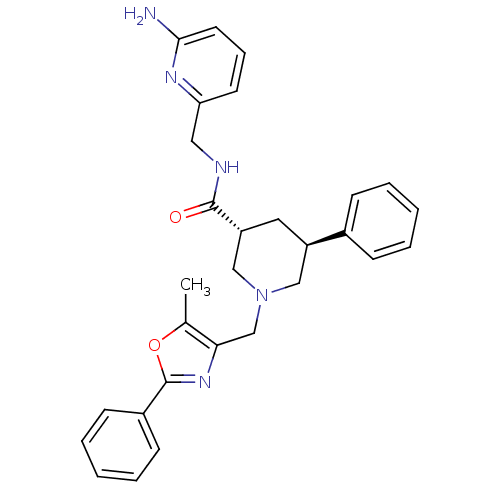
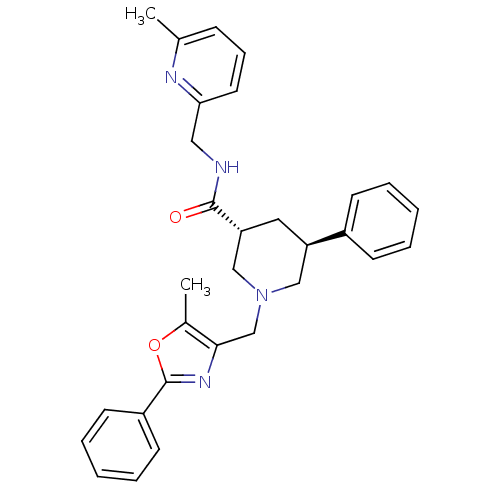
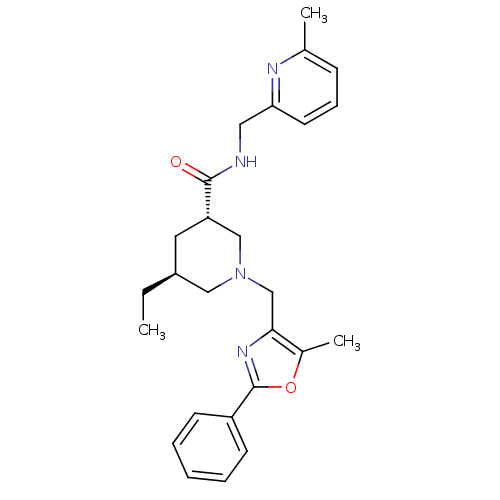
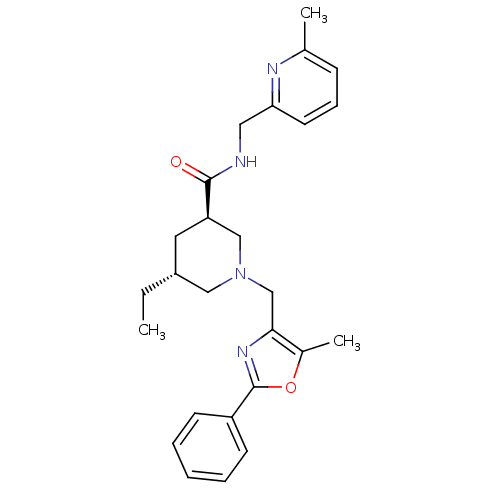
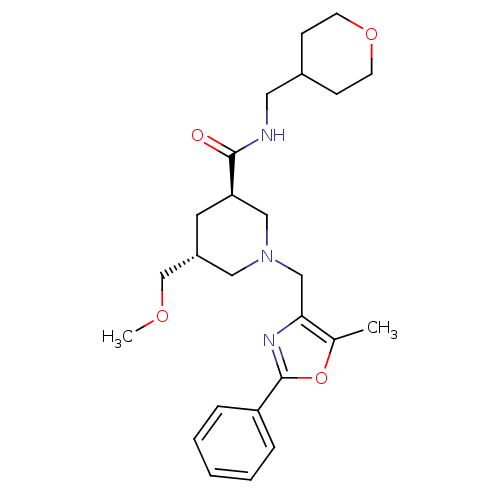
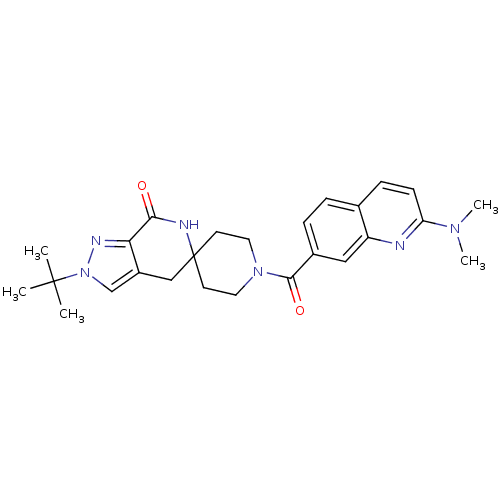
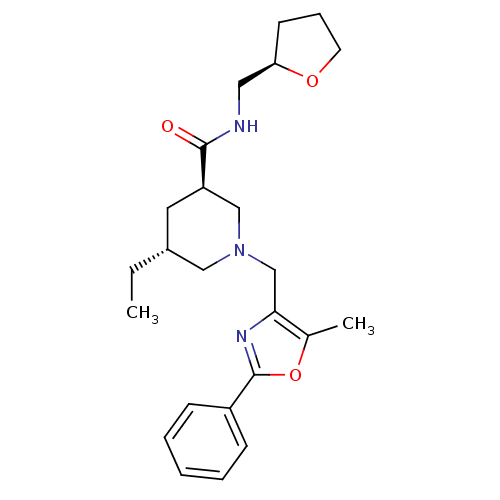
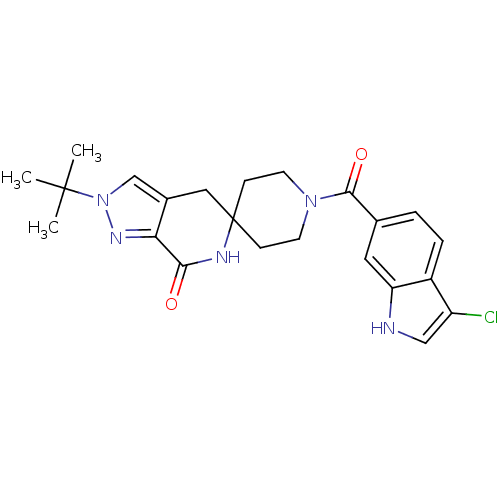
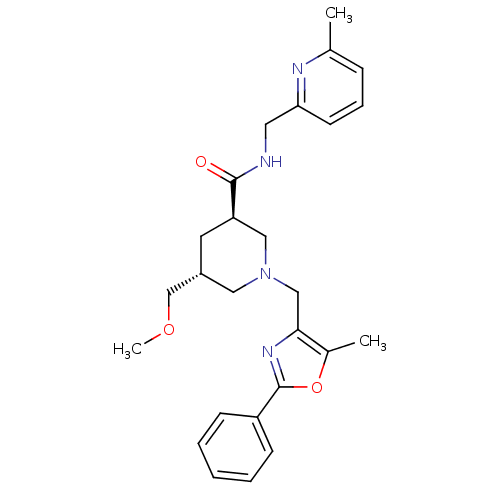
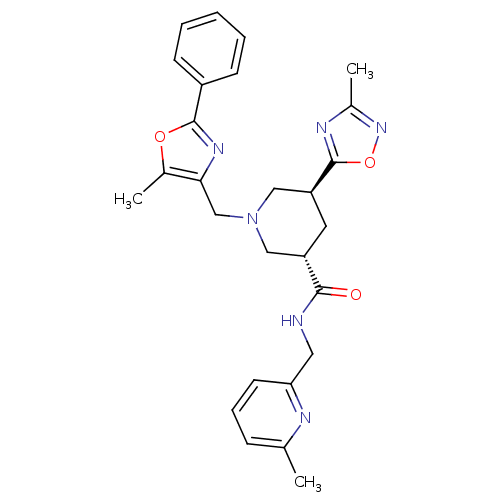
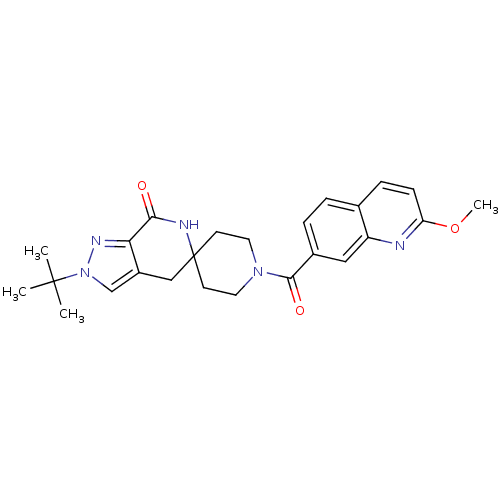
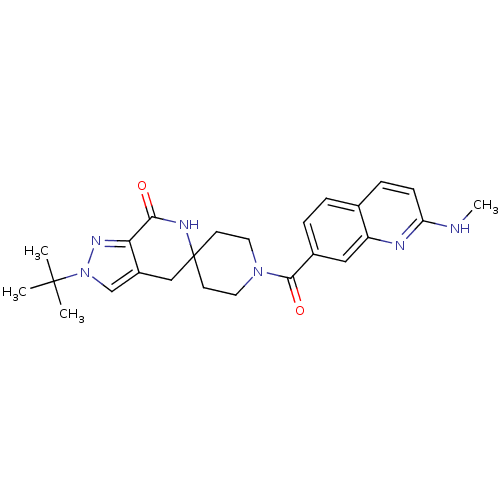
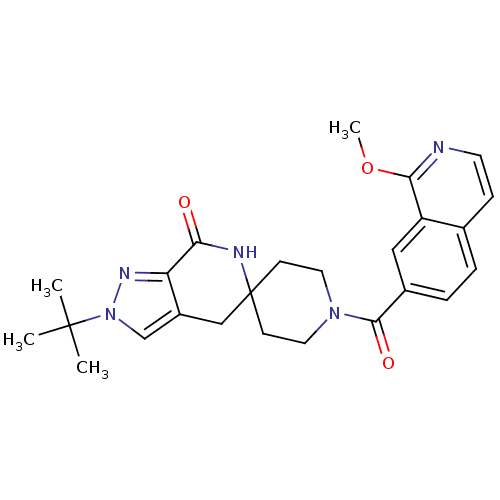
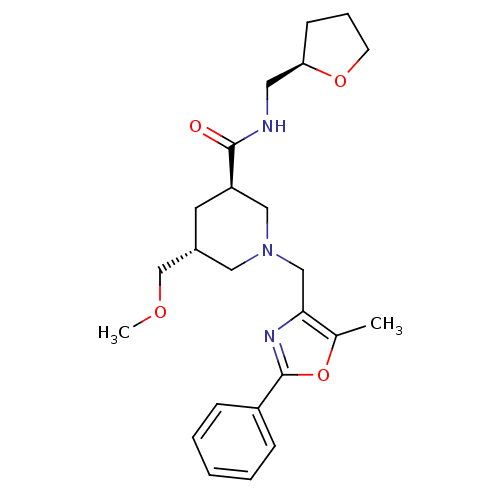
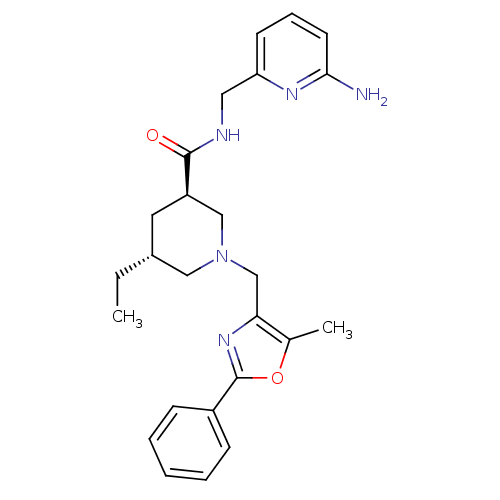
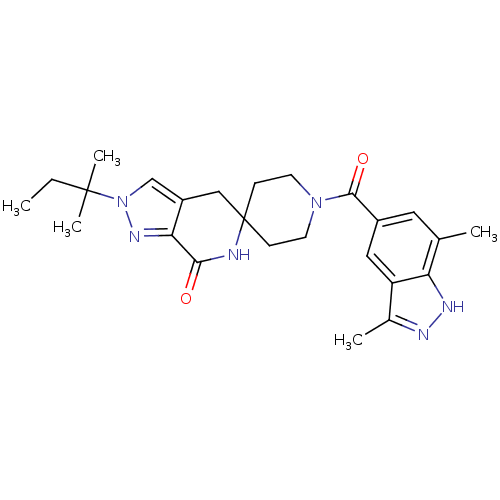
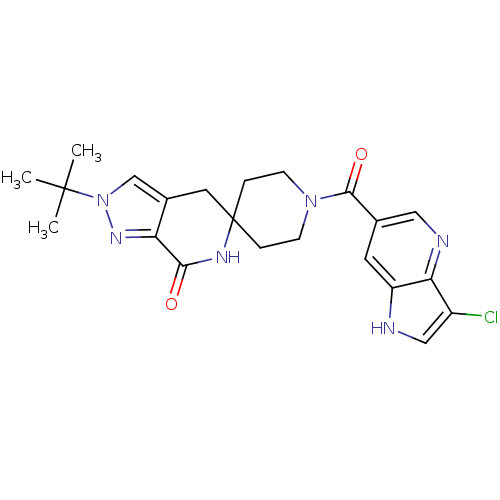
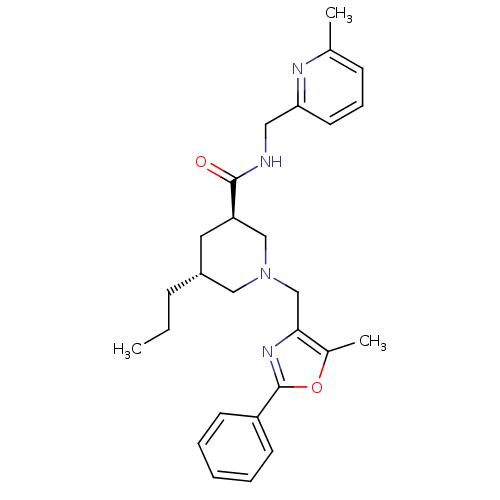
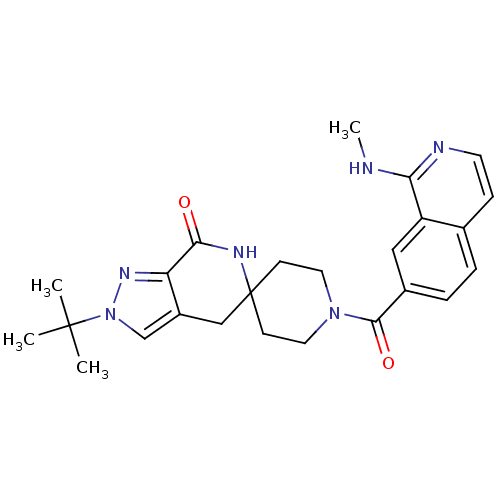
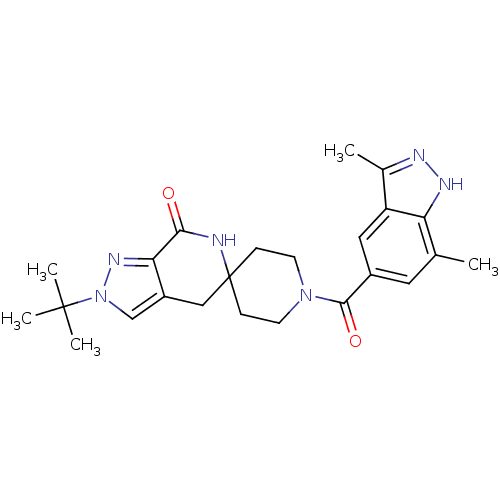
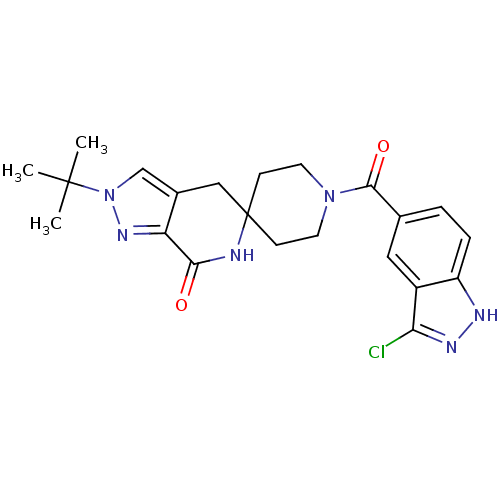
Affinity DataKi: 0.5nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of Cy3B conjugated serotonin analogue binding to human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cell membranes after 60 mi...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 1.18E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Displacement of [3H]isopropyl 4-(1-(4-(methylsulfonyl)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)piperidine-1-carboxylate/tert-butyl 4-(1-(4-(methyl...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of PDE8BMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of PDE8BMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 0.955nMAssay Description:Inhibition of PDE8BMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me...More data for this Ligand-Target Pair