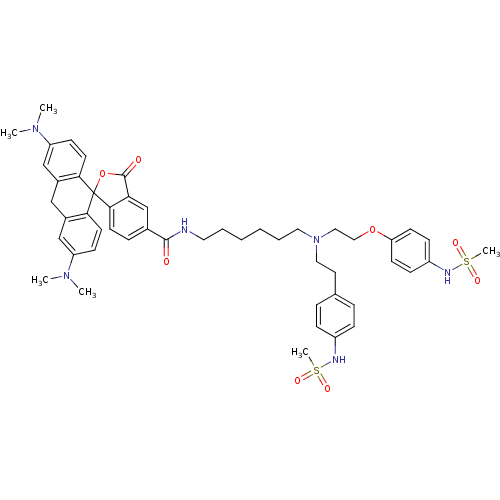
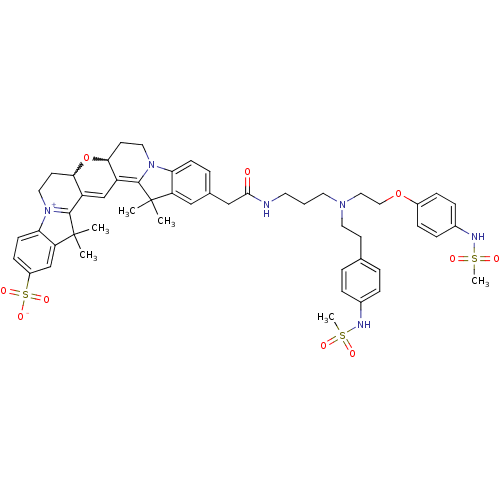
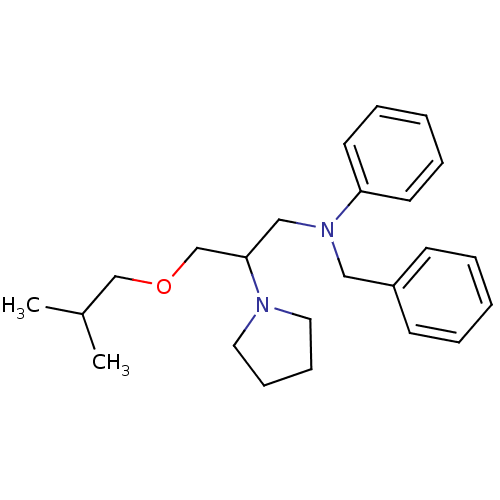
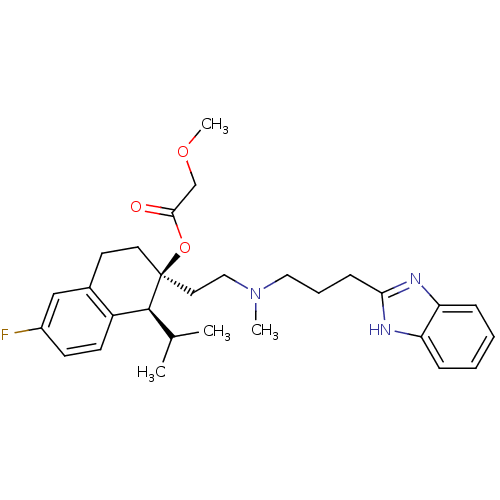
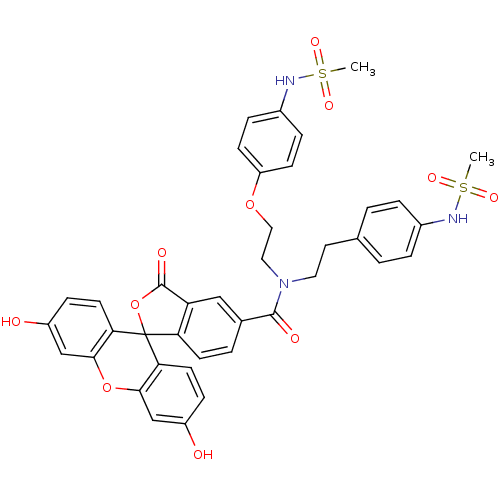
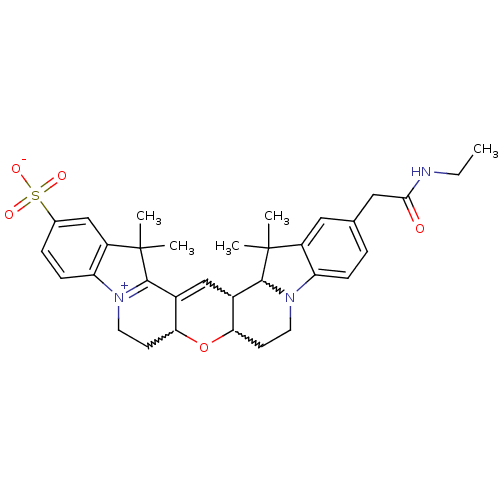
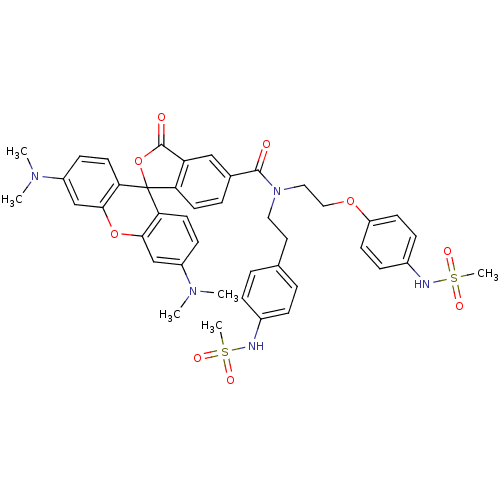
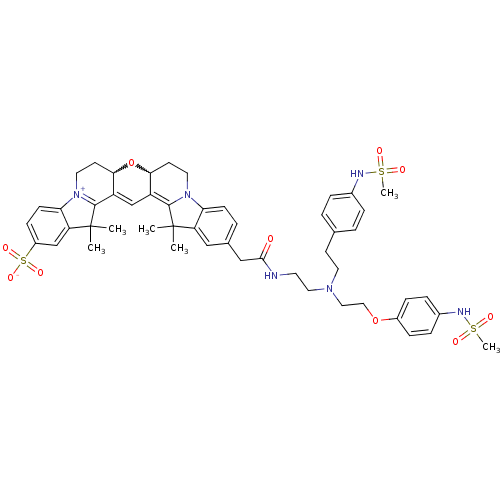
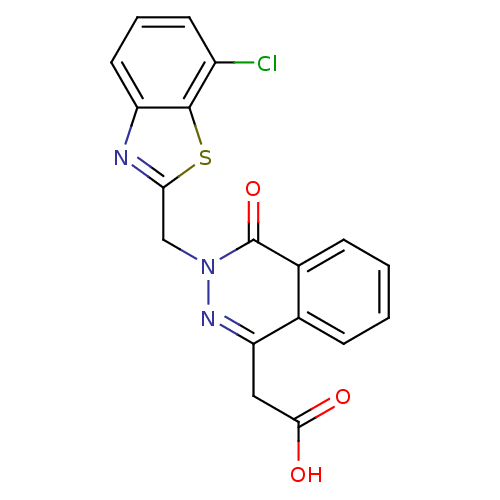
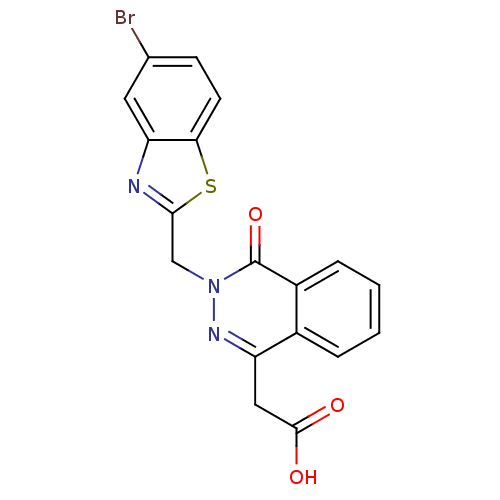
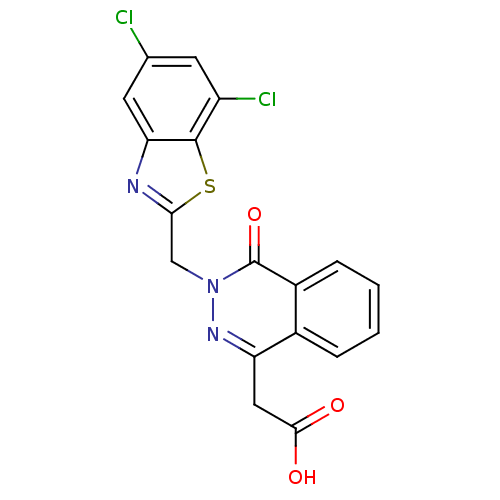
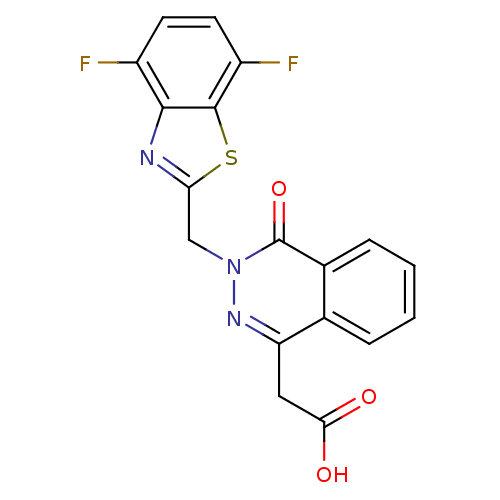
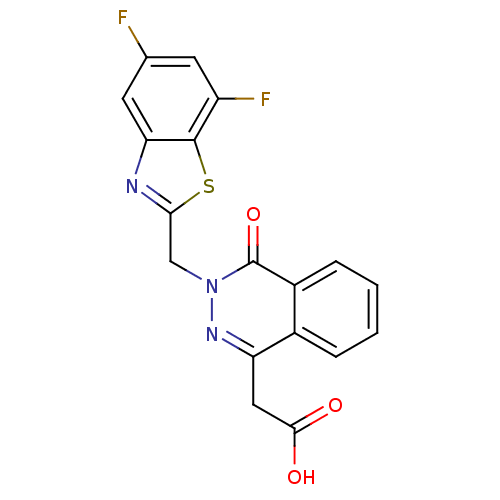
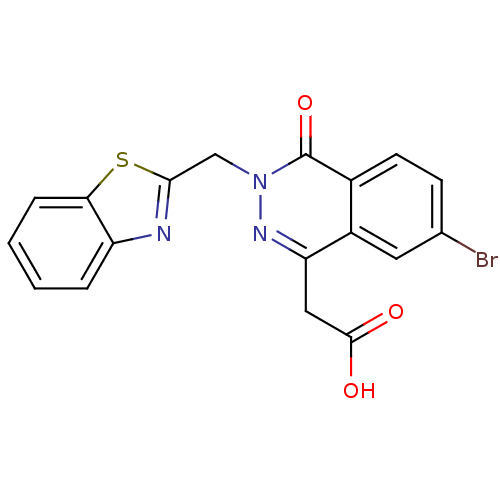
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
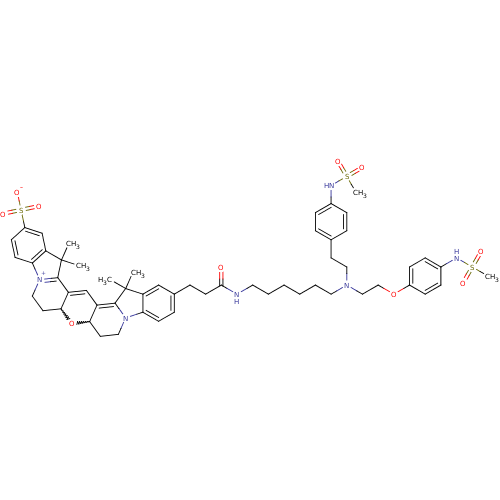
Affinity DataKi: 0.390nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 6.40nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 7.70nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 184nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 190nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 291nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 353nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 980nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 3.08E+3nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 4.06E+3nMAssay Description:Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 7.30E+3nMAssay Description:Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPAMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Compound was tested for the rate of reduction of glyceraldehyde by human placental aldose reductase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Compound was tested for the inhibition of the human placental aldose reductase using the substrate as glyceraldehyde.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
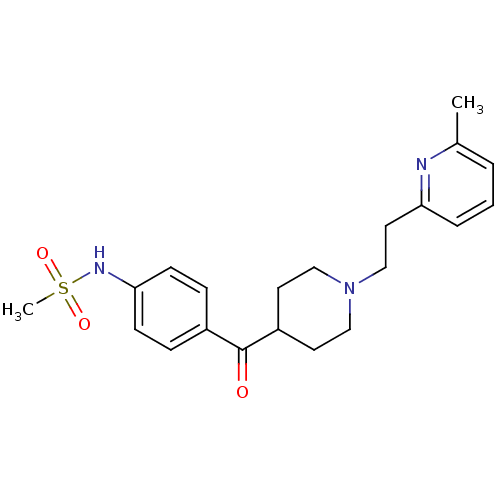
Affinity DataIC50: 3nMAssay Description:Blockade of hERG expressed in HEK293 cells by whole-cell patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Compound was tested for the inhibition of the rat lens aldose reductase using the substrate as glucose.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 7.10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.20nMAssay Description:Blockade of hERG expressed in HEK293 cells by whole-cell patch clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Compound was tested for the inhibition of the human placental aldose reductase using the substrate as glyceraldehyde.More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta.More data for this Ligand-Target Pair

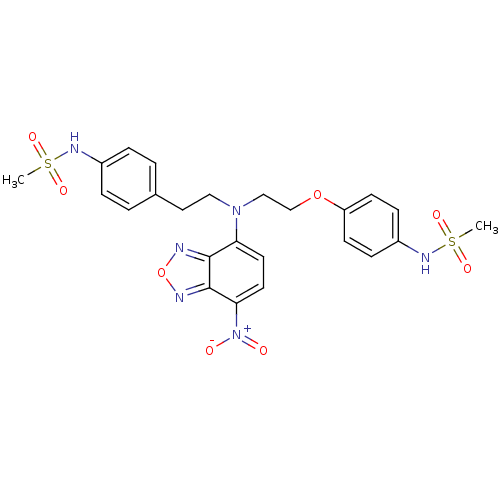


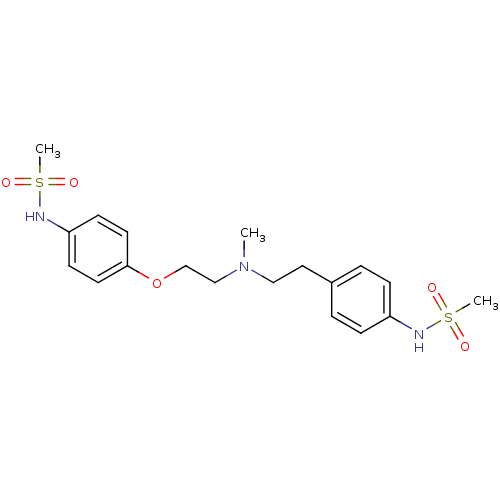
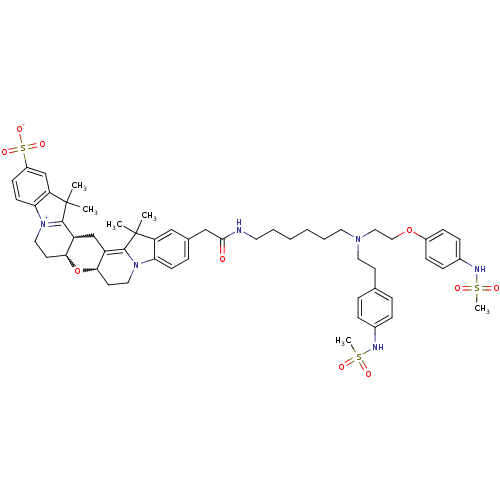
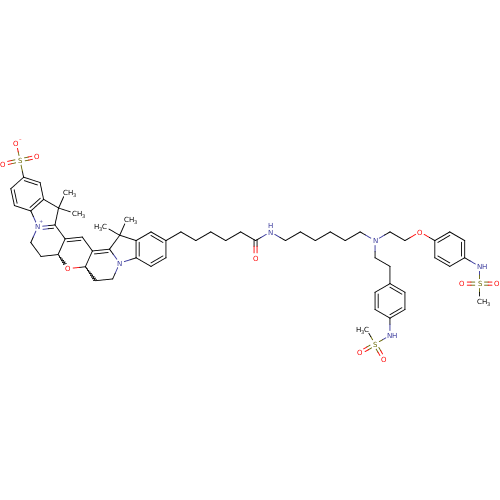

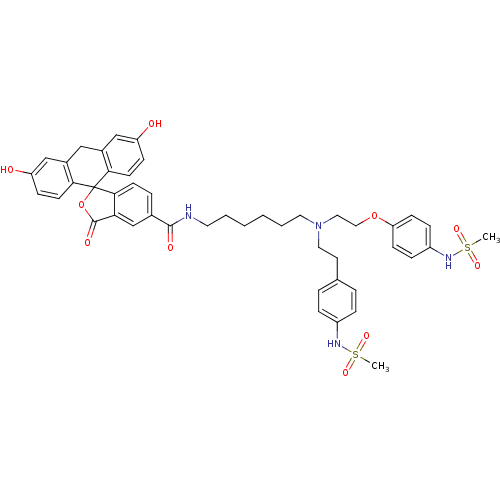
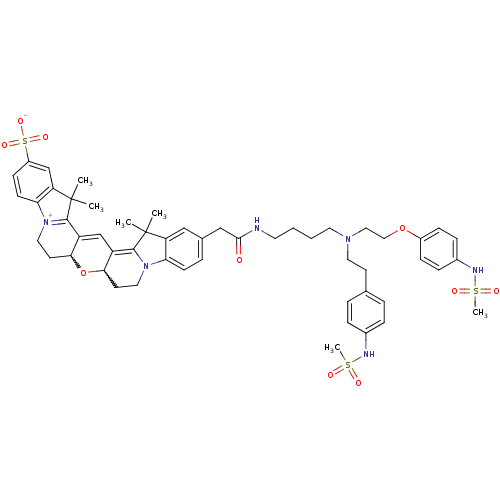

 3D Structure (crystal)
3D Structure (crystal)