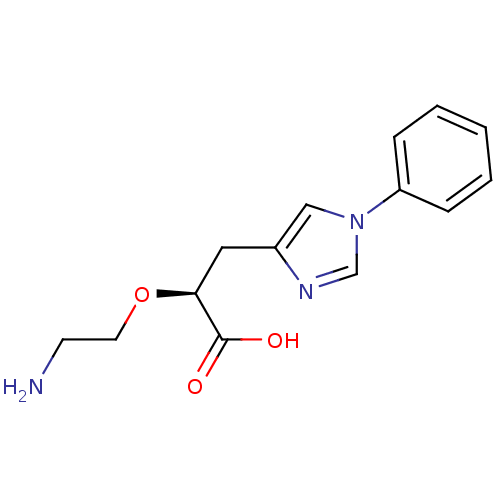
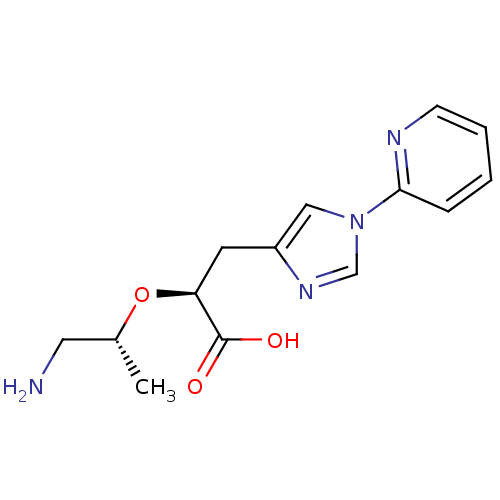
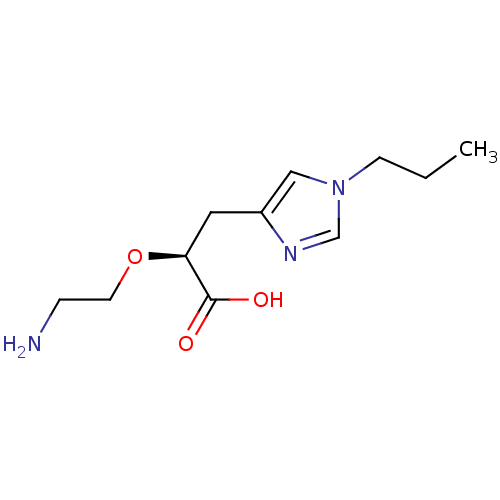
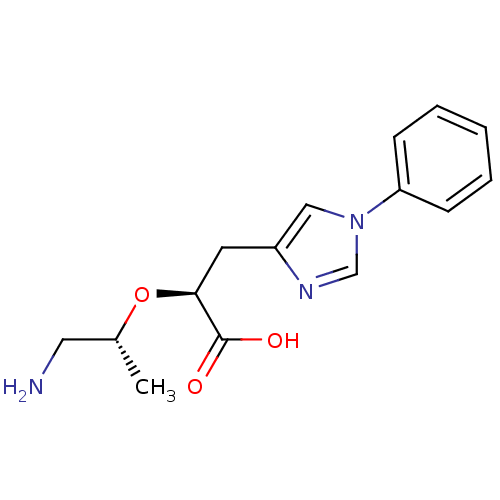
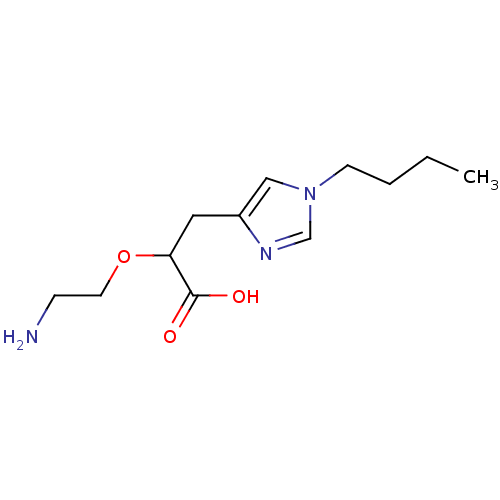
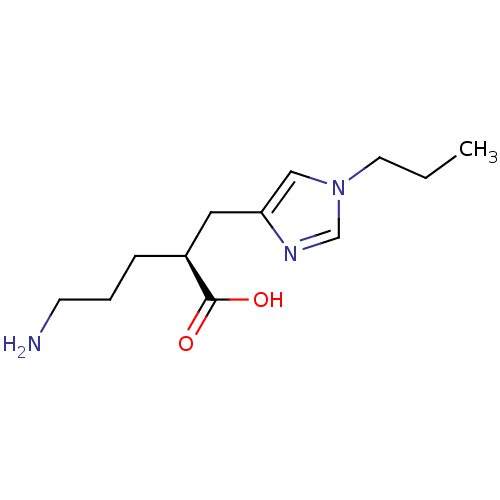
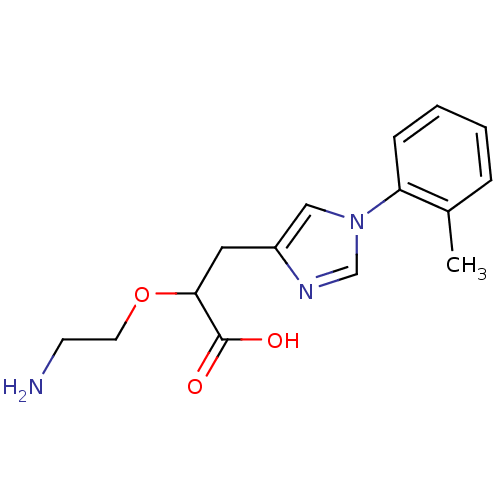
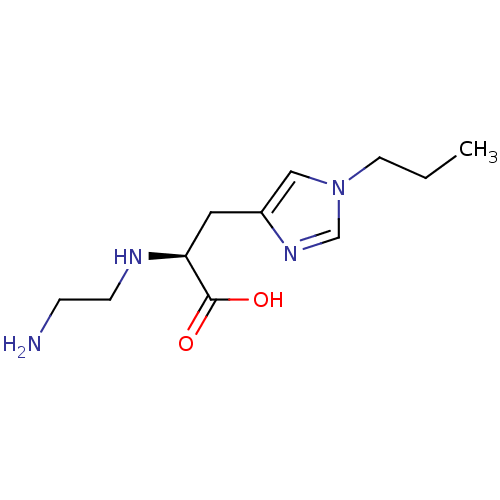
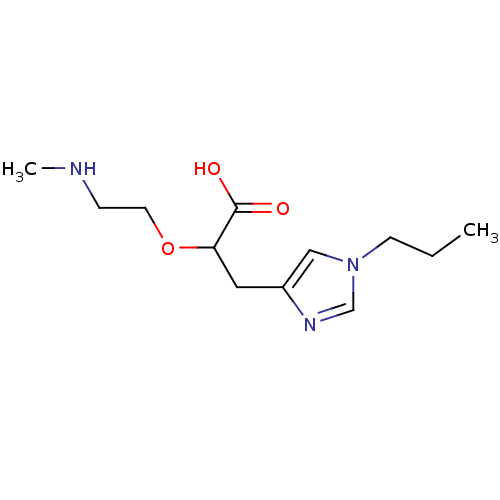
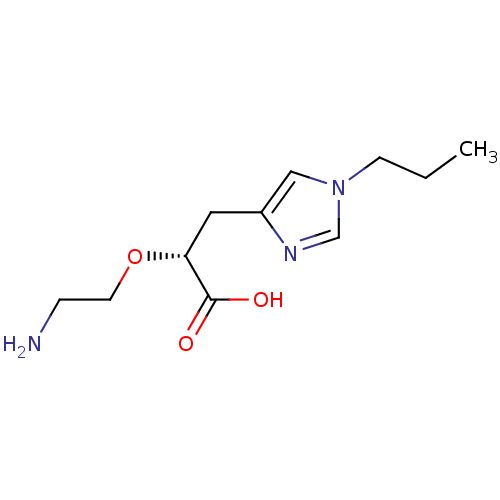
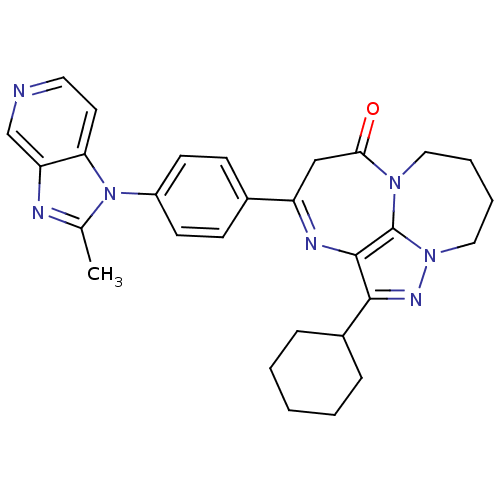
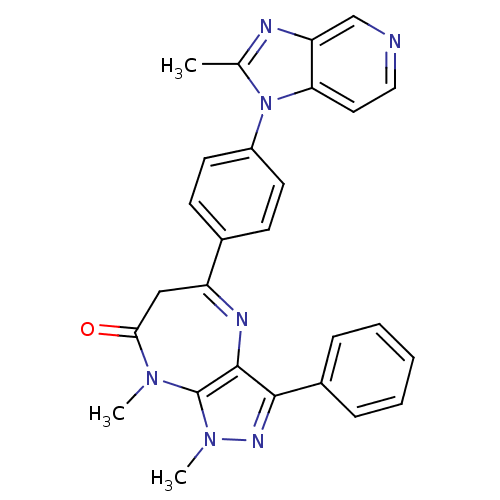
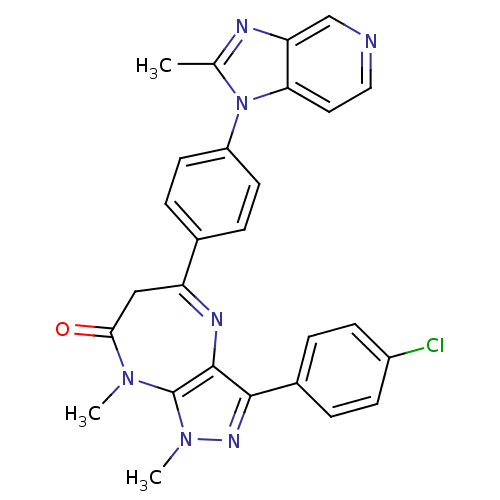
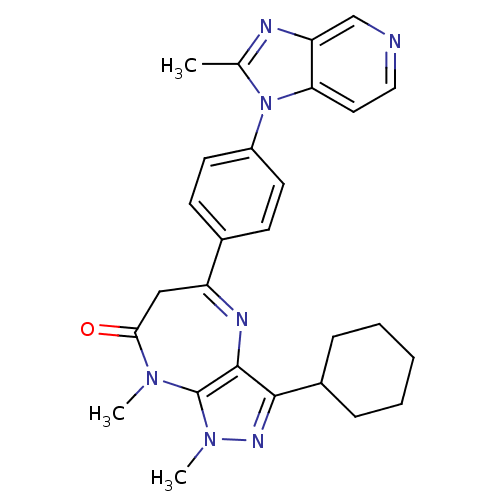
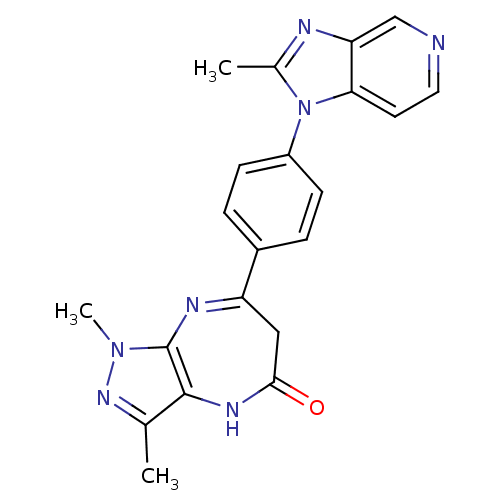
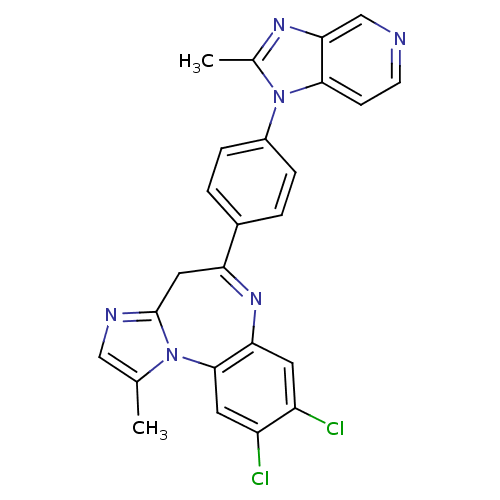
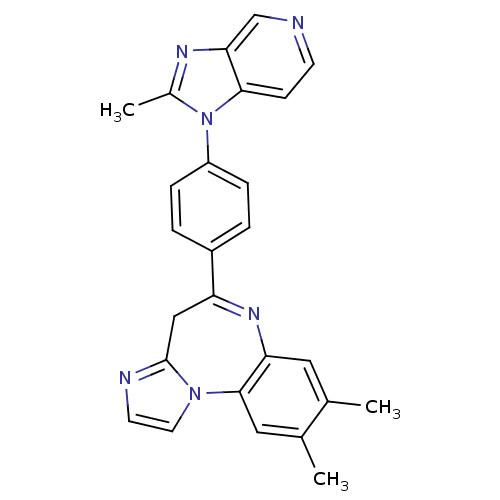
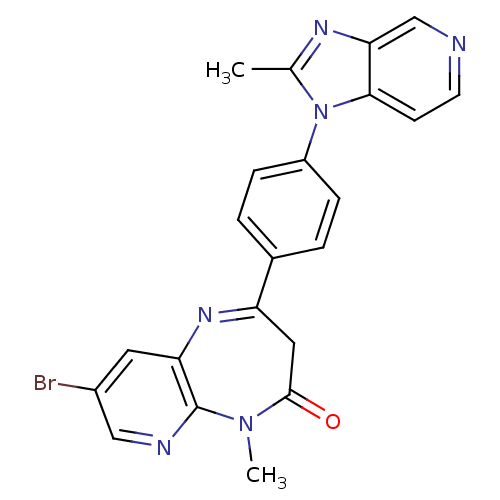
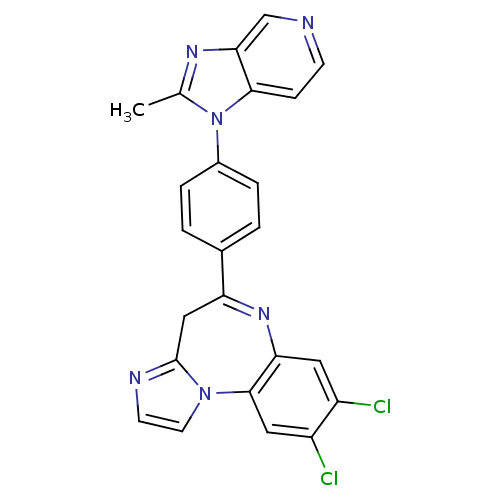
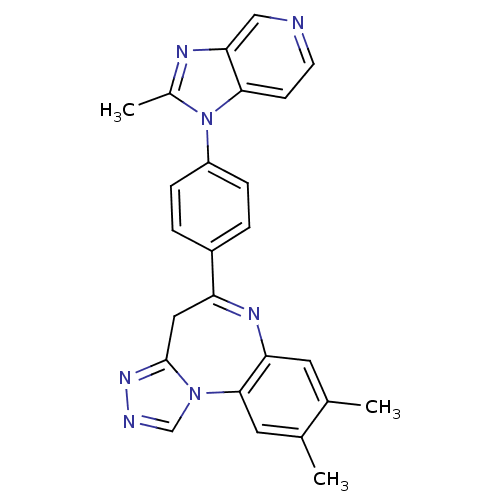
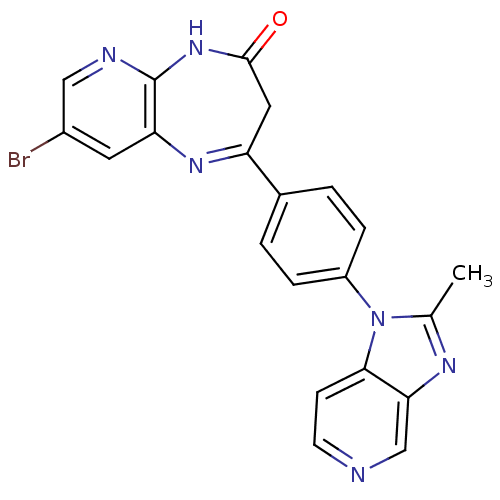
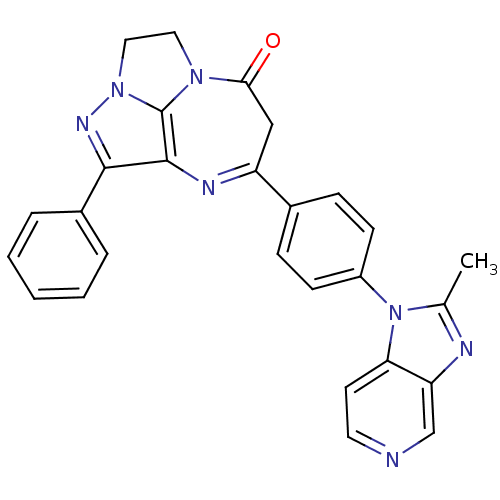
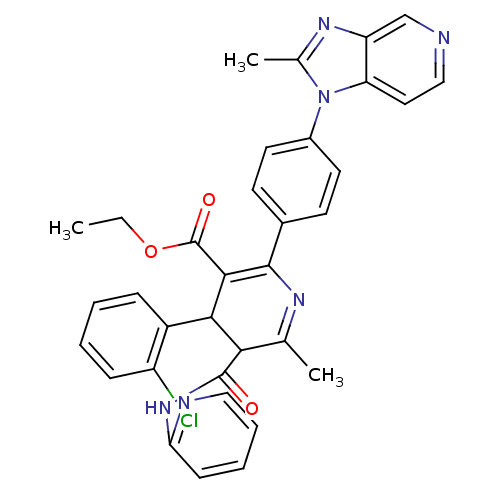
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
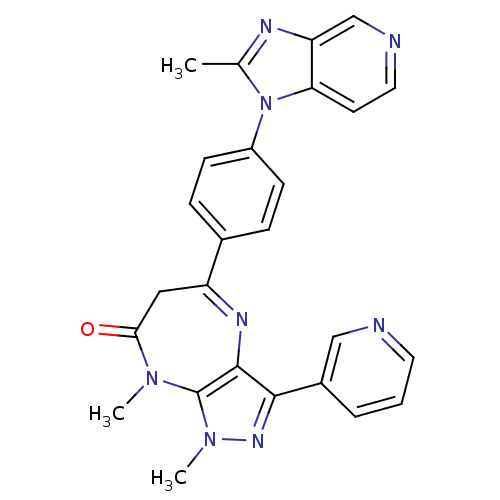
Affinity DataKi: 3.5nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
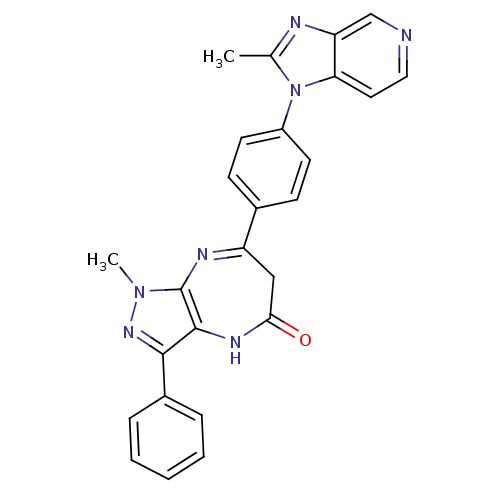
Affinity DataKi: 5.70nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
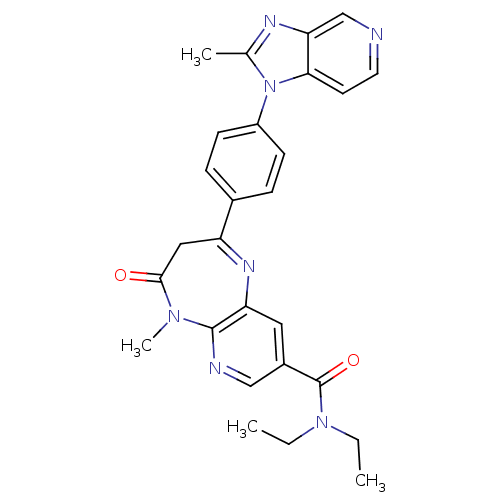
Affinity DataKi: 5.80nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
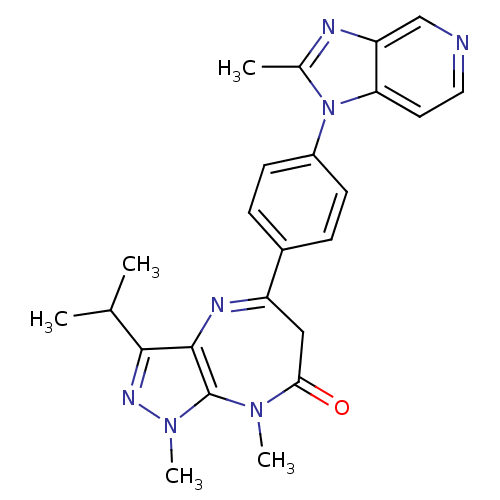
Affinity DataKi: 6.70nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 7.5nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 8.20nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 206nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 238nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 265nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 407nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 minsMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 1.16E+3nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 2.72E+3nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
TargetCarboxypeptidase B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of human pancreatic carboxypeptidase BMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit plateletsMore data for this Ligand-Target Pair