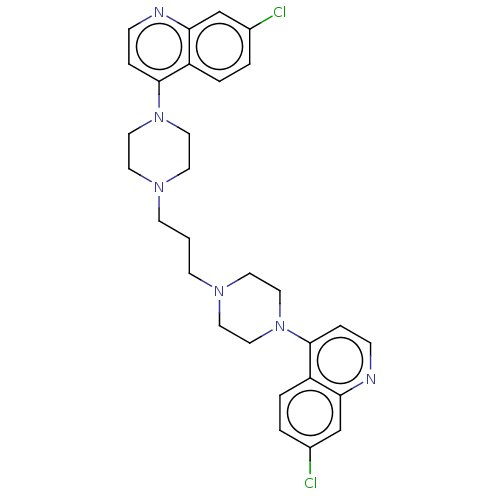
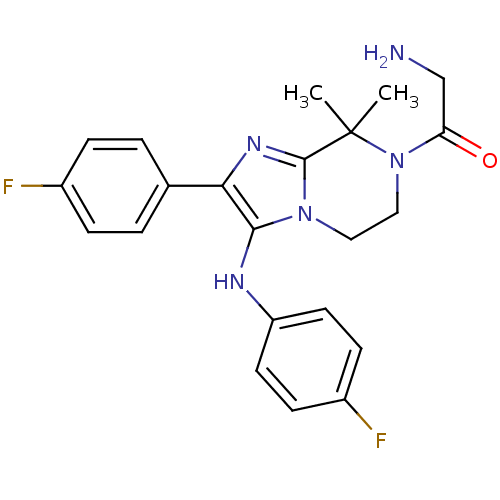
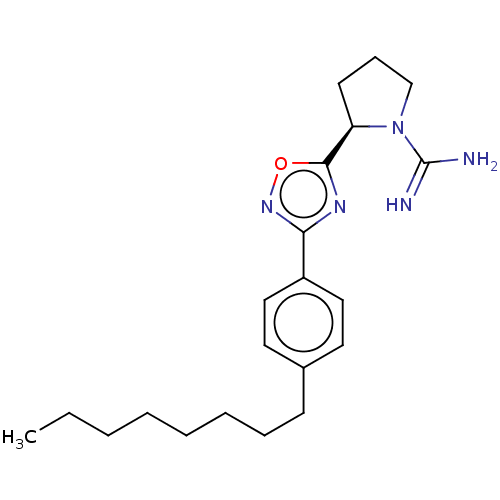
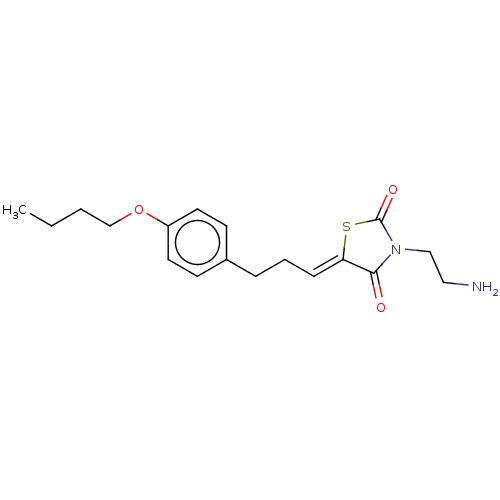
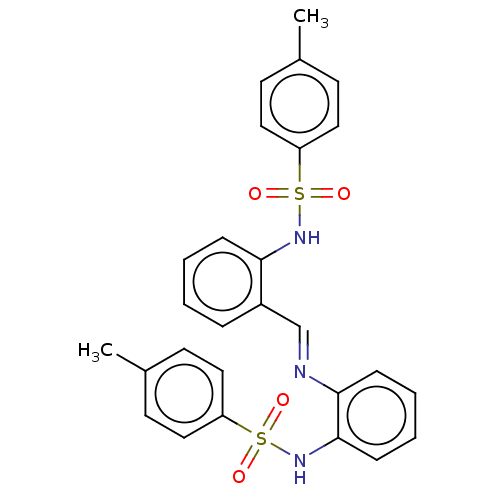
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of SK1 (unknown origin) using 5 uM of sphingosine as substrateMore data for this Ligand-Target Pair
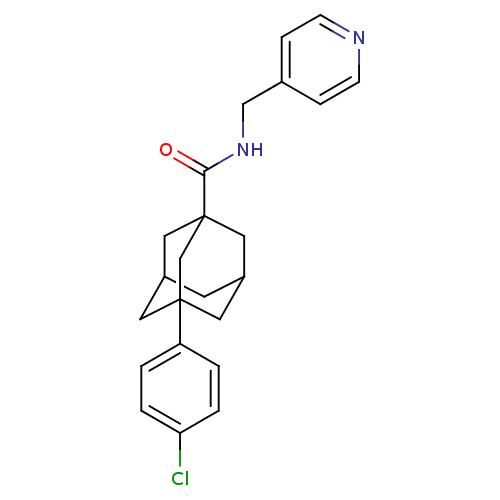
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibition of SK1 (unknown origin)More data for this Ligand-Target Pair
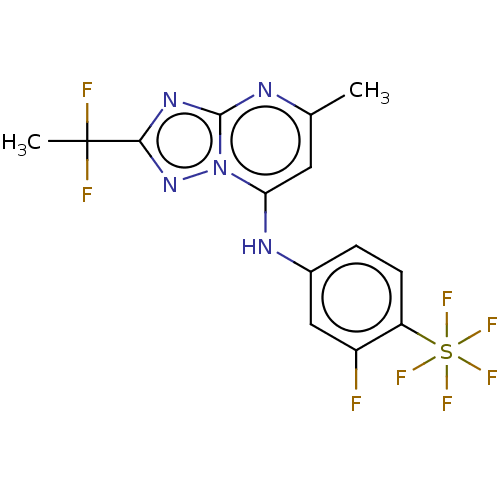
TargetCytochrome P450 3A4(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
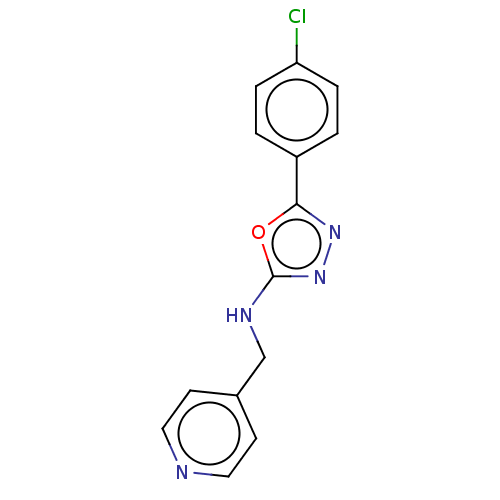
TargetCytochrome P450 3A4(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataKi: 156nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of recombinant SK2 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gam...More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of SK2 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 6.40E+3nMAssay Description:Inhibition of recombinant SK2 (unknown origin) using sphingosine as substrate and gamma[32P]ATP by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of human SK2 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 7.90E+3nMAssay Description:Inhibition of SK2 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of SK2 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.30E+4nMAssay Description:Inhibition of recombinant SK1 (unknown origin) expressed in Sf9 cells assessed as [33P]S1P formation using D-erythro sphingosine as substrate and gam...More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of SK1 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataKi: 2.70E+4nMAssay Description:Inhibition of human SK1 using D-erythro sphingosine as substrate and gamma[33P]ATP by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of SK1 (unknown origin) using 3 uM of sphingosine as substrateMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Plasmodium falciparum (isolate 3D7))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of Plasmodium falciparum DHODH expressed in Escherichia coli using L-dihydroorotate as substrateMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Plasmodium falciparum (isolate 3D7))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of Plasmodium falciparum DHODH expressed in Escherichia coli using L-dihydroorotate as substrateMore data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of SK1 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam...More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 148nMAssay Description:Inhibition of SK2 (unknown origin)More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 387nMAssay Description:Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 1.58E+3nMAssay Description:Inhibition of human ERG by patch clamp methodMore data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam...More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ...More data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human DHODH expressed in Escherichia coli using L-dihydroorotate as substrateMore data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of recombinant human SK2 assessed as production of [32P] S1P using 10 uM sphingosine as substrate by TLC method in presence of 100 uM [gam...More data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of SK2 (unknown origin)More data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Nav1.5 (unknown origin)More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cav1.2 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetSphingosine kinase 2(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of SK1 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as reduction in testosterone 6beta-hydroxylation activity by high-pressure liquid chromatogra...More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
The Walter and Eliza Hall Institute of Medical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes assessed as reduction in midazolam 1-hydroxylase activity by . high-pressure liquid chromatography-tan...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes assessed as reduction in midazolam 1-hydroxylase activity by . high-pressure liquid chromatography-tan...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes assessed as reduction in midazolam 1-hydroxylase activity by . high-pressure liquid chromatography-tan...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes assessed as reduction in midazolam 1-hydroxylase activity by . high-pressure liquid chromatography-tan...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes assessed as reduction in midazolam 1-hydroxylase activity by . high-pressure liquid chromatography-ta...More data for this Ligand-Target Pair
TargetSphingosine kinase 1(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of recombinant His-tagged human SK1 assessed as production of [32P]-S1P using 10 uM sphingosine as substrate by TLC method in presence of ...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)