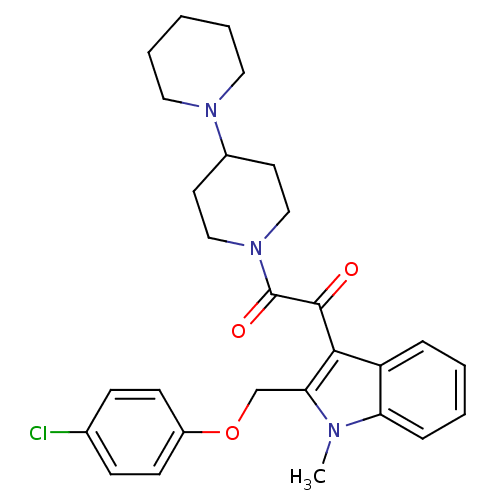
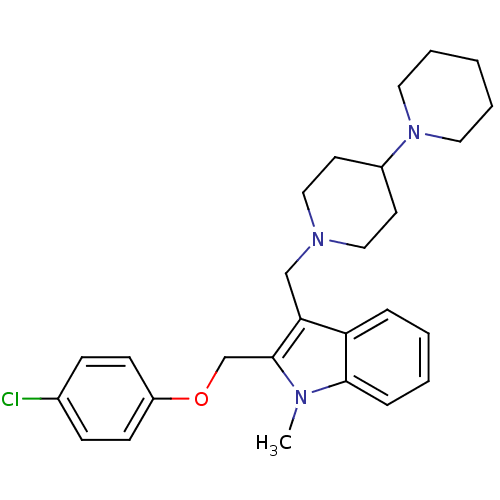
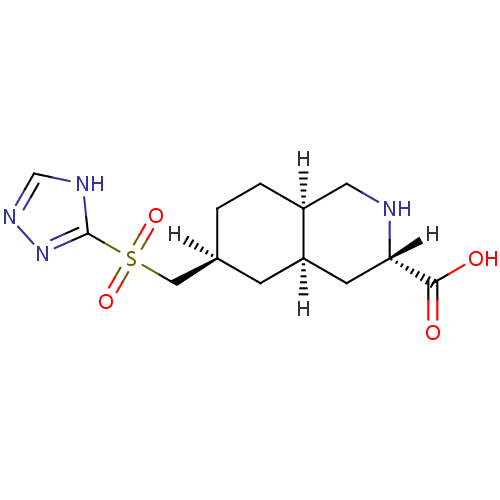
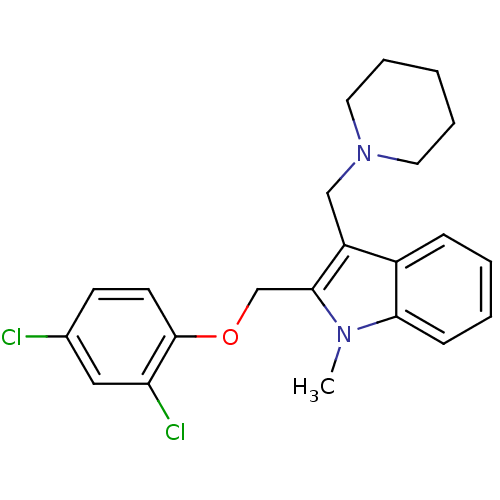
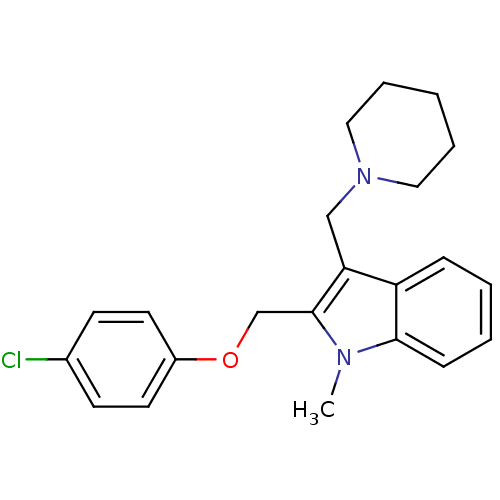
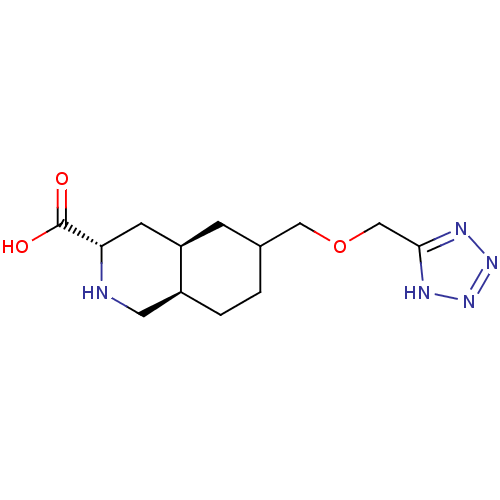
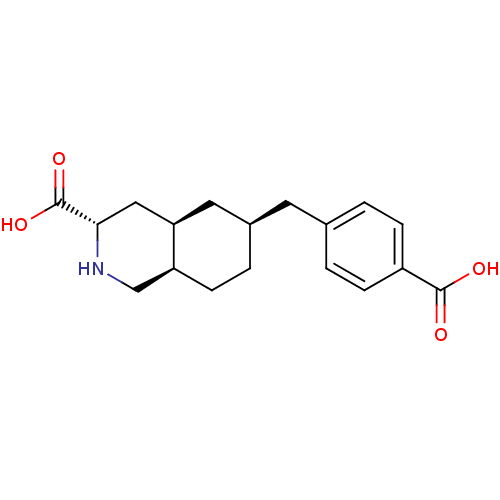
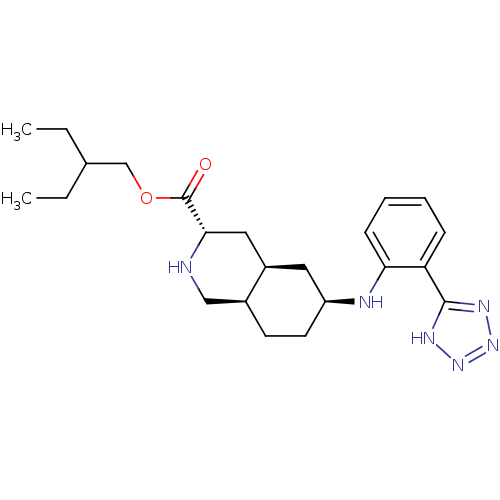
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.75nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Ability of the compound to reverse Neuropeptide Y receptor type 1-induced inhibition of forskolin-induced inhibition of forskolin-stimulated cAMPMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 4.60nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 26nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 93nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 98nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 440nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 559nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.26E+3nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
Affinity DataKi: 1.60E+3nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.32E+3nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.32E+3nMAssay Description:Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cellsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
Affinity DataKi: 3.20E+3nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
TargetGlutamate receptor ionotropic, kainate 3(Homo sapiens (Human))
Eli Lilly and Company
Curated by PDSP Ki Database
Eli Lilly and Company
Curated by PDSP Ki Database
Affinity DataKi: 9.20E+3nMAssay Description:Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 2More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 2More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 4(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 5More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 2More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Affinity for human Neuropeptide Y receptor type 5More data for this Ligand-Target Pair


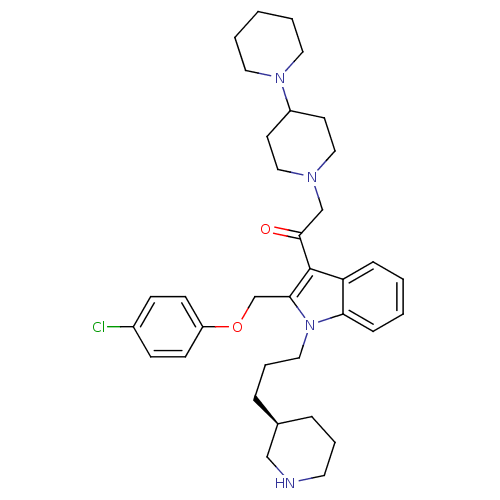


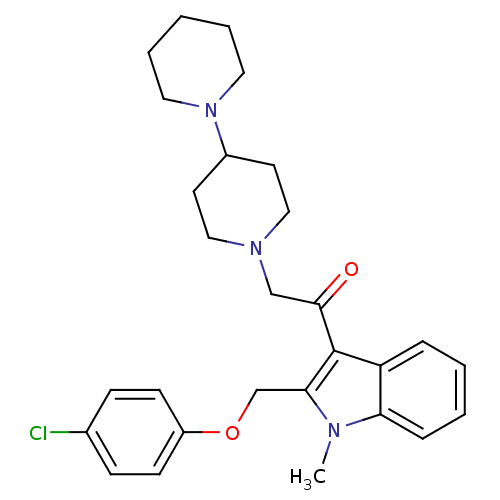
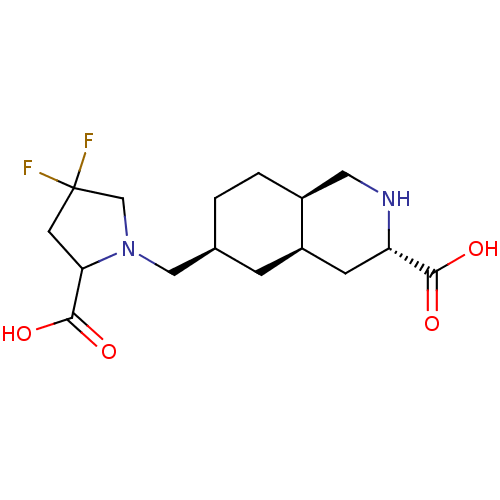
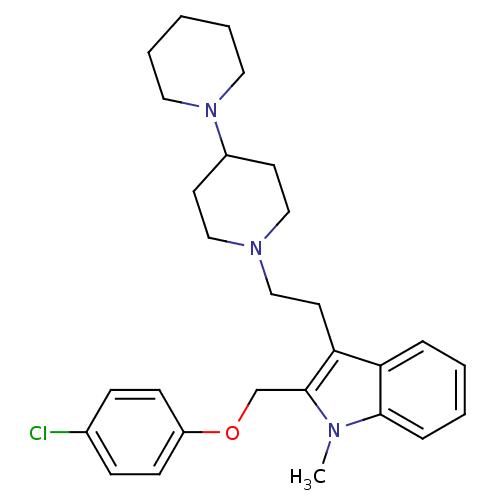


 3D Structure (crystal)
3D Structure (crystal)