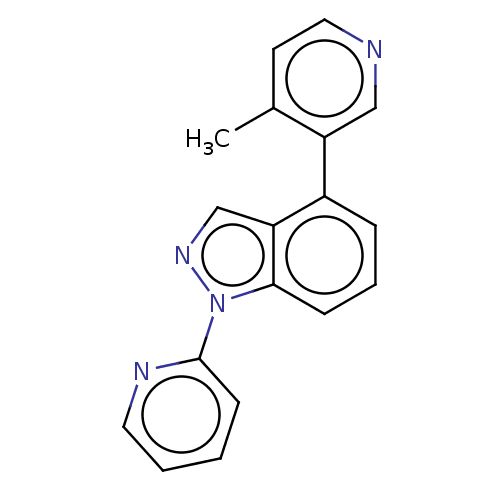
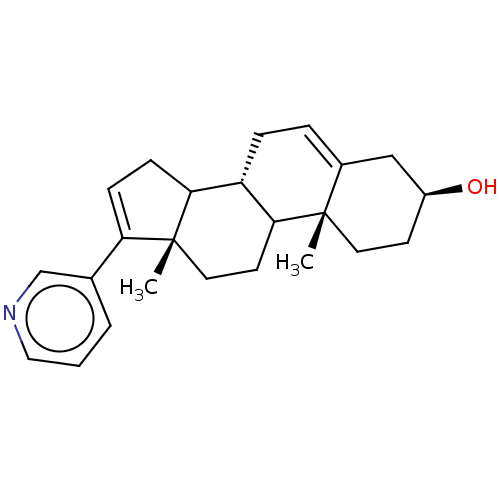
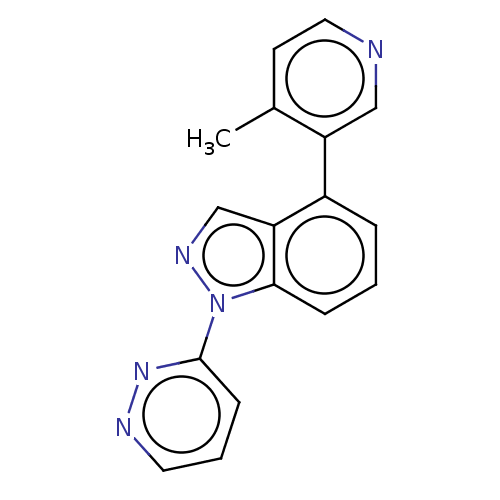
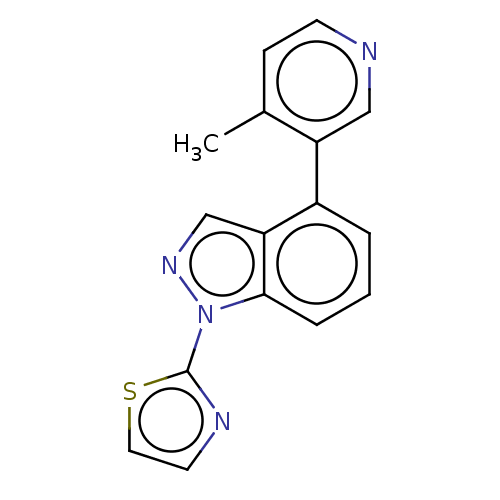
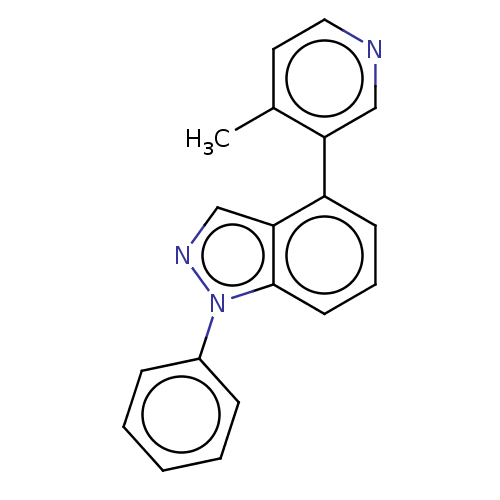
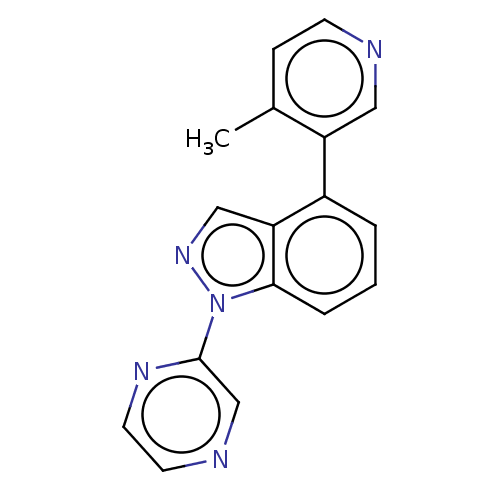
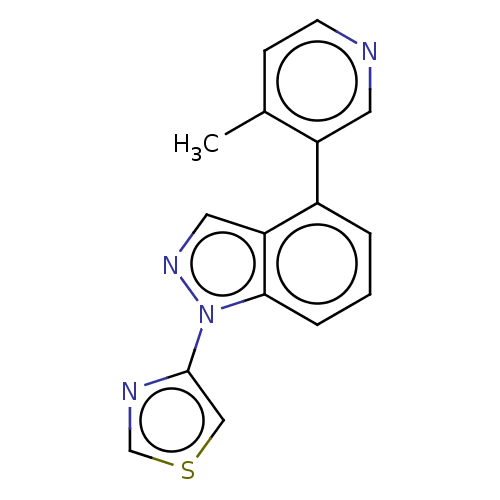
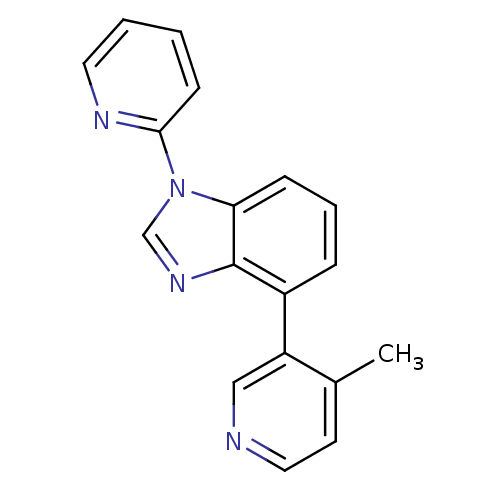
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Macaca fascicularis)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Macaca fascicularis)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Macaca fascicularis)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Macaca fascicularis)
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of CYP17A1 in human adrenal microsomes using [3H]-pregnenolone as substrate incubated for 90 mins by scintillation proximity assay in pres...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of CYP17A1 in human adrenal microsomes using [3H]-pregnenolone as substrate incubated for 90 mins by scintillation proximity assay in pres...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 75nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 94nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 119nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 872nMAssay Description:Inhibition of CYP11B1 in human H295R cells using deoxycorticosterone as substrate incubated for 48 hrs by LC-MS methodMore data for this Ligand-Target Pair
TargetSteroid 21-hydroxylase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.05E+3nMAssay Description:Inhibition of CYP21A2 in human H295R cells using hydroxy progesterone as substrate incubated for 120 mins by LC-MS methodMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetSteroid 21-hydroxylase(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of CYP21A2 in human H295R cells using hydroxy progesterone as substrate incubated for 120 mins by LC-MS methodMore data for this Ligand-Target Pair
TargetCytochrome P450 11B1, mitochondrial(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of CYP11B1 in human H295R cells using deoxycorticosterone as substrate incubated for 48 hrs by LC-MS methodMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair