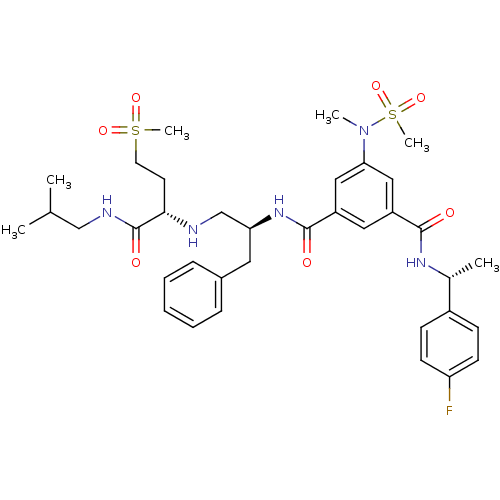
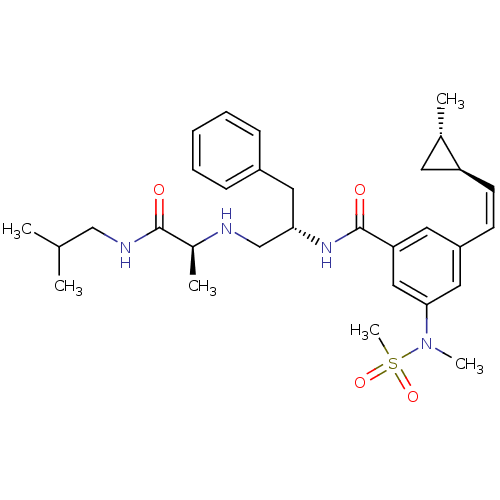
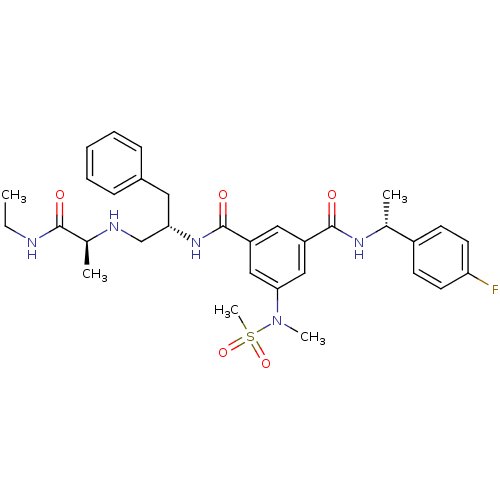
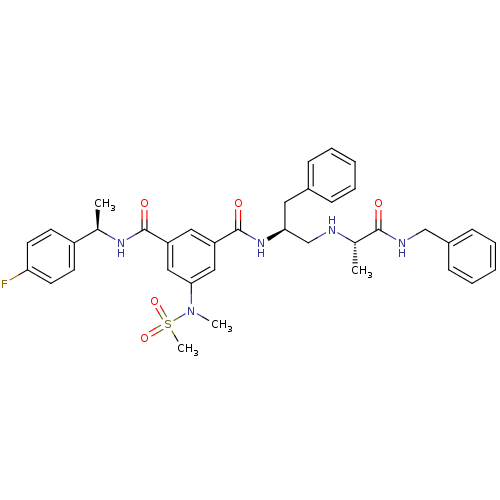
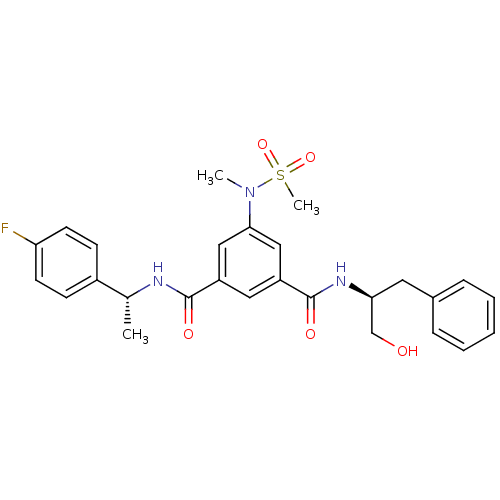
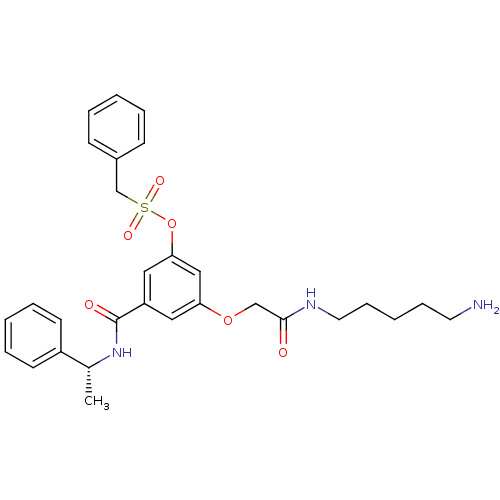
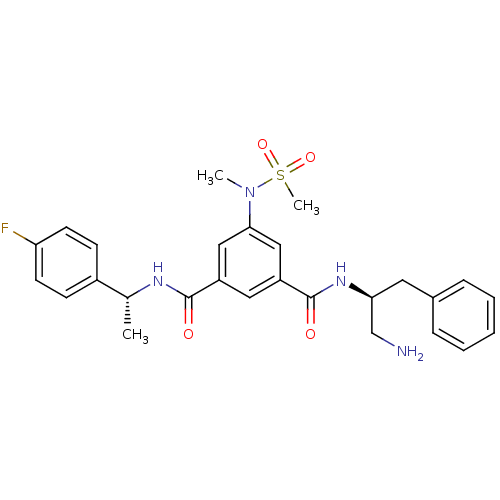
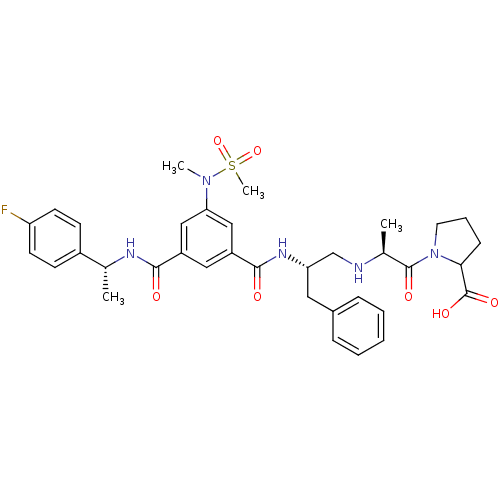
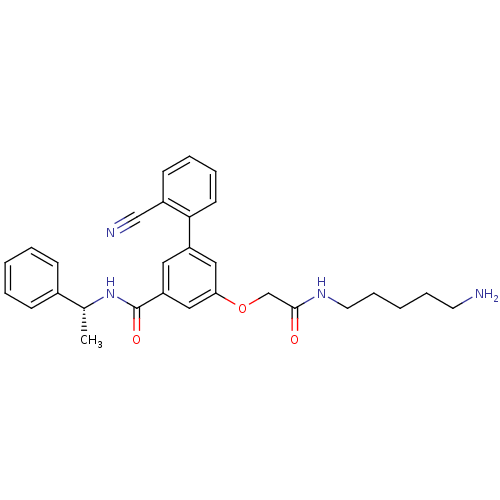
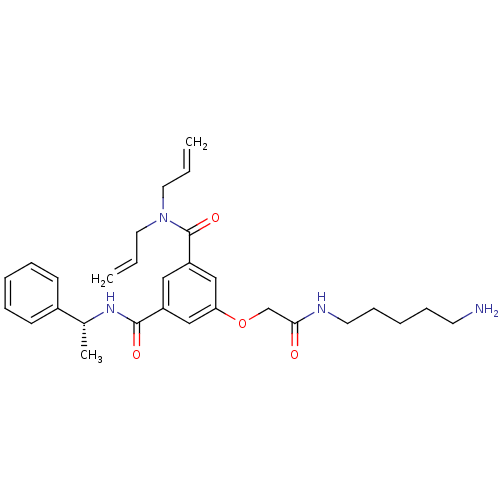
Affinity DataKi: 0.0840nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.360nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:The compound was tested for its ability to inhibit thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 510nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 580nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 770nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 860nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 1.27E+4nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+4nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 1.01E+5nMAssay Description:The compound was tested for its ability to inhibit trypsin.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 117nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 139nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 630nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 4.5 T: 2°CAssay Description:The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ...More data for this Ligand-Target Pair

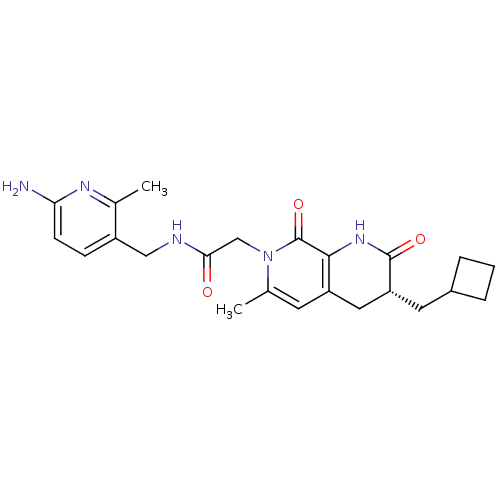
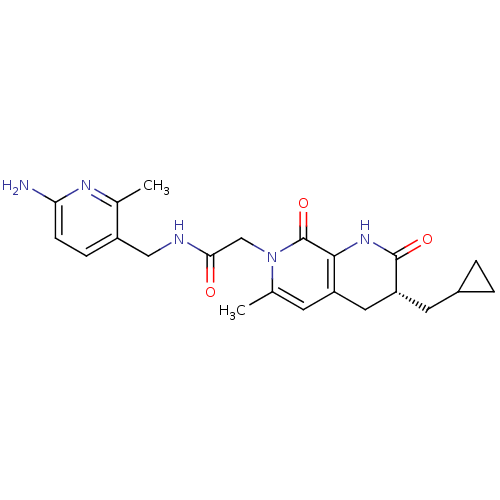
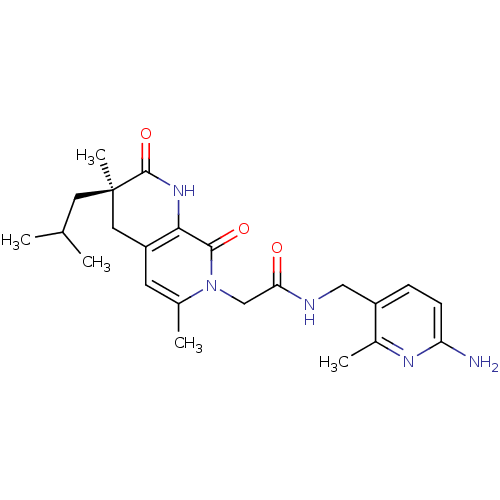
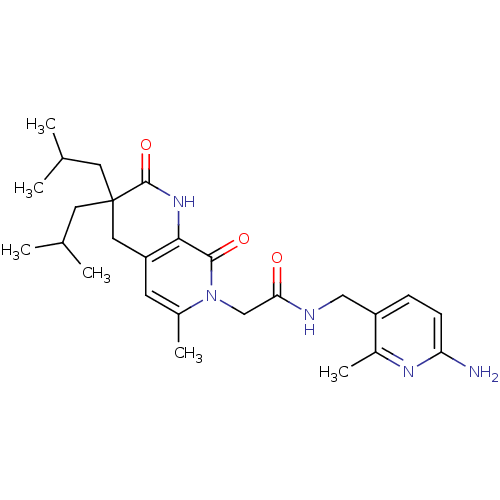
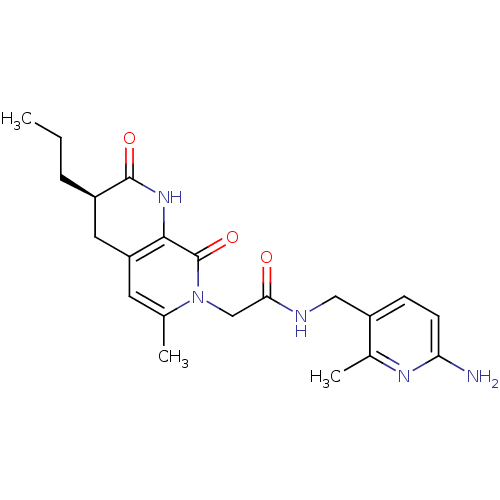
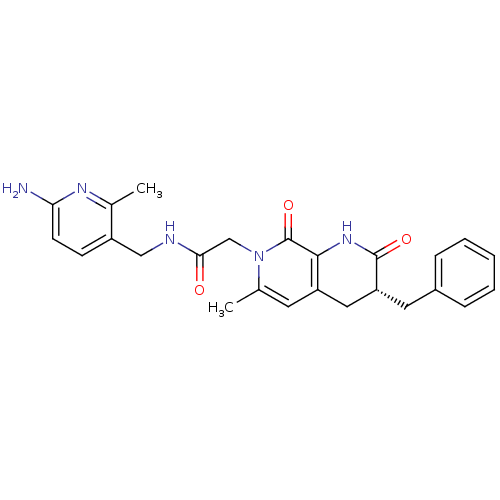

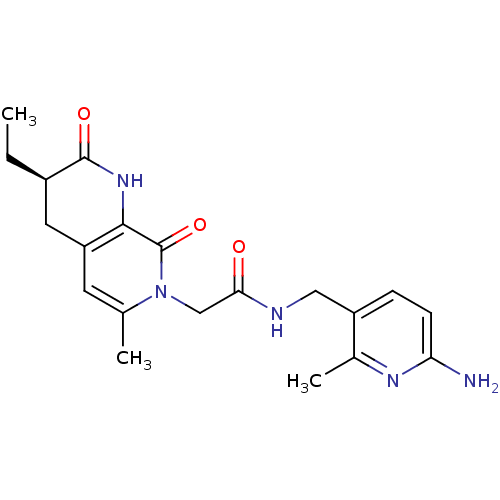
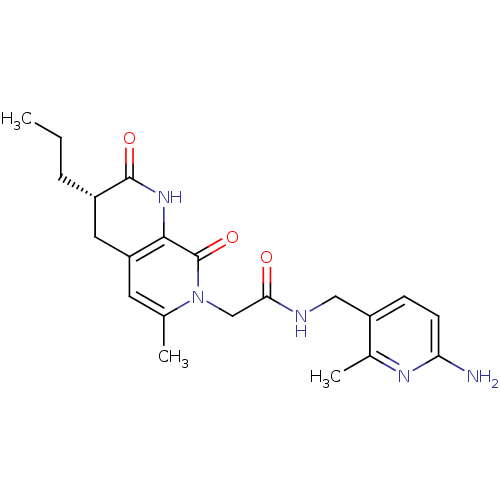
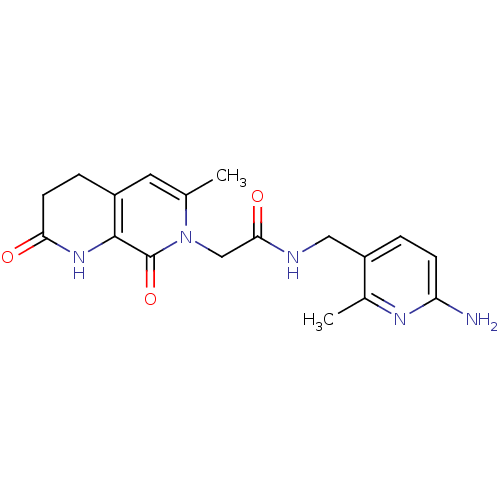
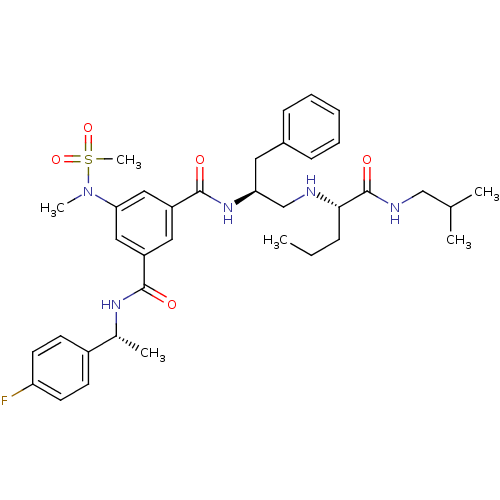

 3D Structure (crystal)
3D Structure (crystal)