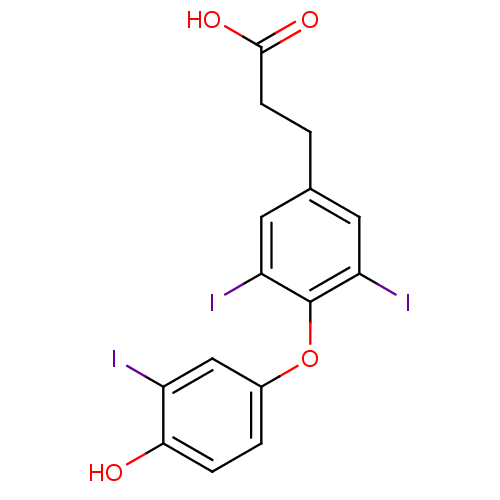
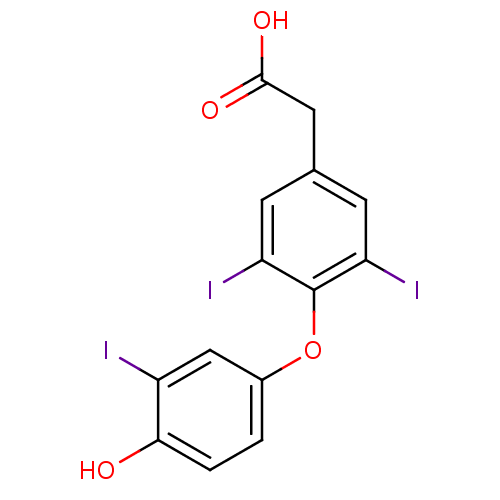
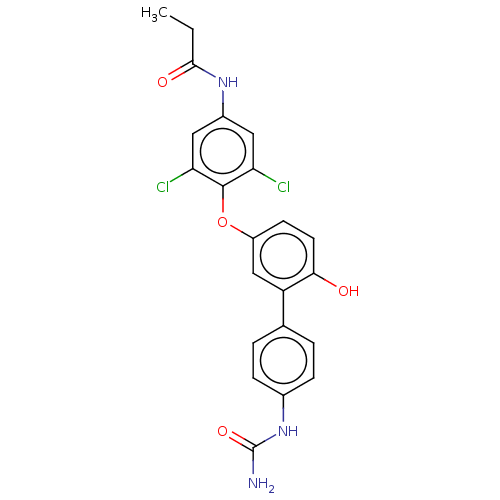
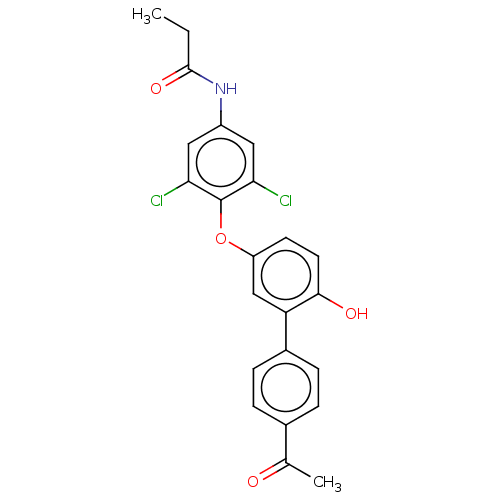
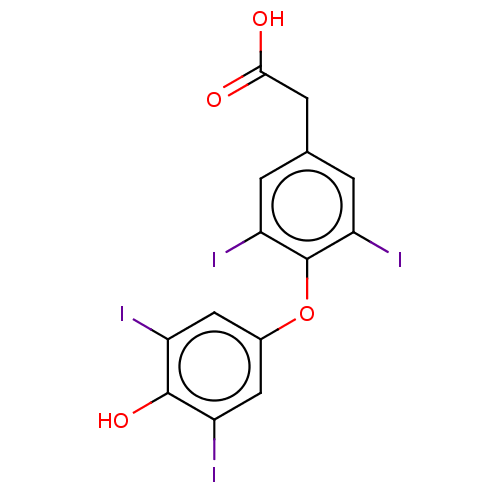
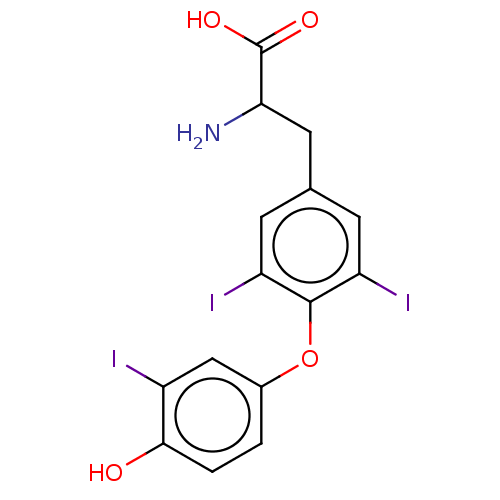
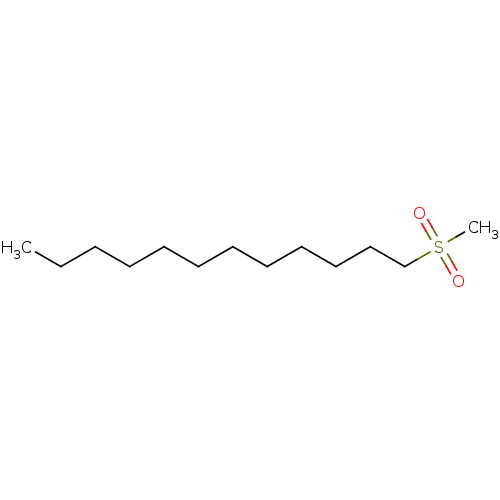
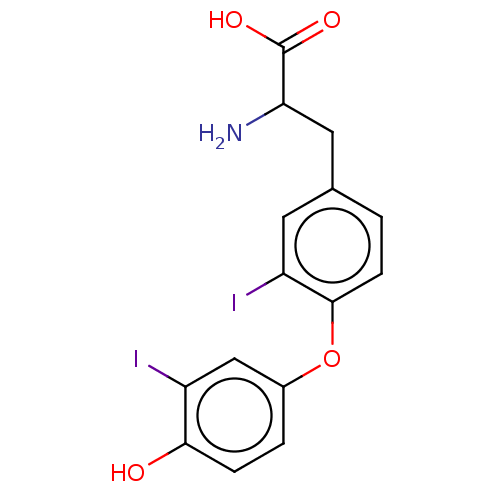
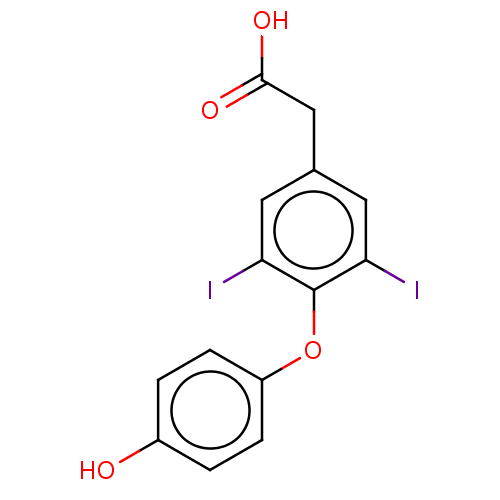
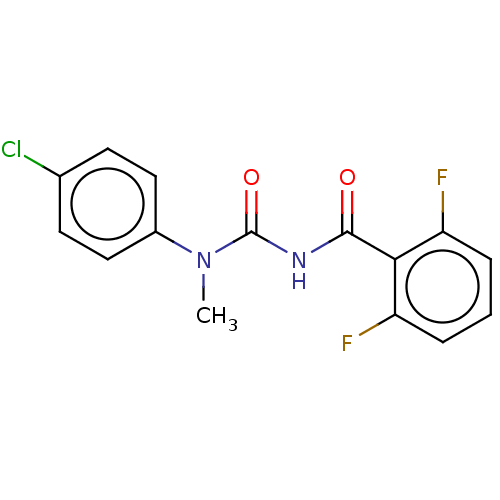
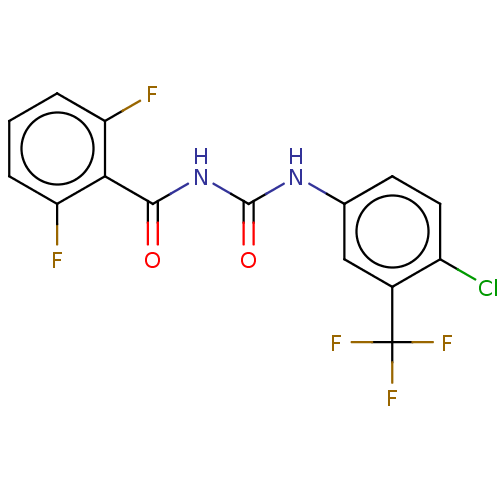
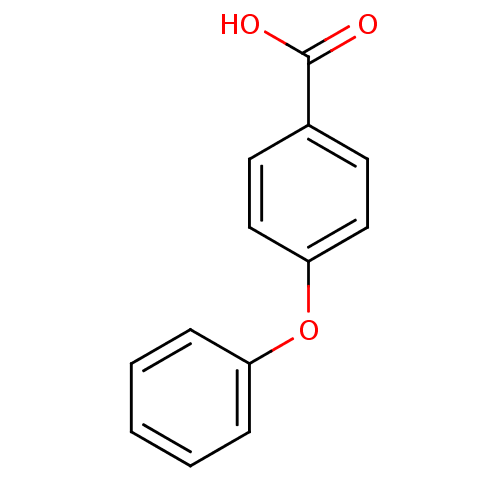
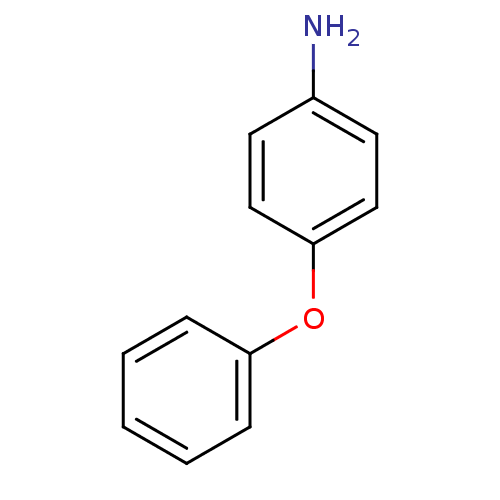
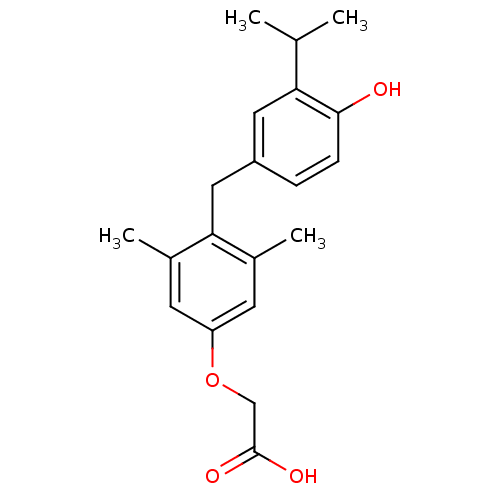
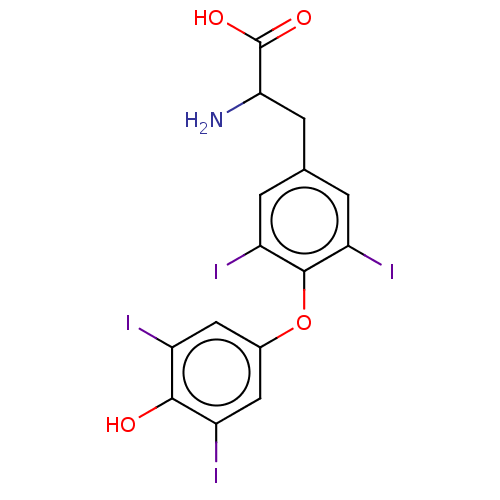
Affinity DataKi: 779nMAssay Description:Fluorescence polarization (FP) data were measured on a microplate reader (Tecan Infinite M1000, Mannedorf, Switzerland) at 27° C. For Kd measurements...More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nMAssay Description:Fluorescence polarization (FP) data were measured on a microplate reader (Tecan Infinite M1000, Mannedorf, Switzerland) at 27° C. For Kd measurements...More data for this Ligand-Target Pair
Affinity DataKi: 1.92E+3nMAssay Description:Fluorescence polarization (FP) data were measured on a microplate reader (Tecan Infinite M1000, Mannedorf, Switzerland) at 27° C. For Kd measurements...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of recombinant human sEH expressed in insect High Five cells preincubated for 5 mins followed by addition of t-DPPO as substrate measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of recombinant human mEH expressed in baculovirus expression system assessed as formation of 6-methoxy-2-naphthaldehyde preincubated for 5...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of recombinant human sEH expressed in insect High Five cells preincubated for 5 mins followed by addition of t-DPPO as substrate measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70E+3nMAssay Description:Inhibition of recombinant human sEH expressed in insect High Five cells preincubated for 5 mins followed by addition of t-DPPO as substrate measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.60E+3nMAssay Description:Inhibition of recombinant human sEH expressed in insect High Five cells preincubated for 5 mins followed by addition of t-DPPO as substrate measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMAssay Description:Inhibition of recombinant human mEH expressed in baculovirus expression system assessed as formation of 6-methoxy-2-naphthaldehyde preincubated for 5...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+4nMAssay Description:Inhibition of recombinant human mEH expressed in baculovirus expression system assessed as formation of 6-methoxy-2-naphthaldehyde preincubated for 5...More data for this Ligand-Target Pair
Affinity DataIC50: 3.33E+4nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+4nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 9.78E+4nMAssay Description:Inhibition of recombinant human mEH expressed in baculovirus expression system assessed as formation of 6-methoxy-2-naphthaldehyde preincubated for 5...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:The first step in characterizing the two compounds identified by high throughput screening was to verify that the inhibition was reproducible using f...More data for this Ligand-Target Pair

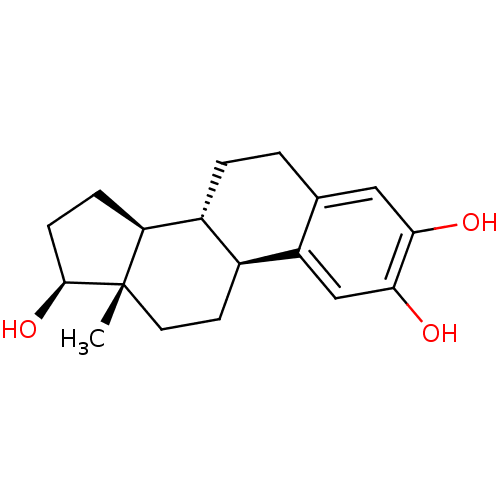
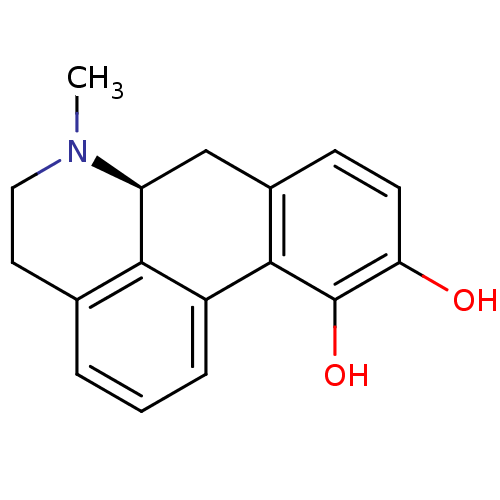
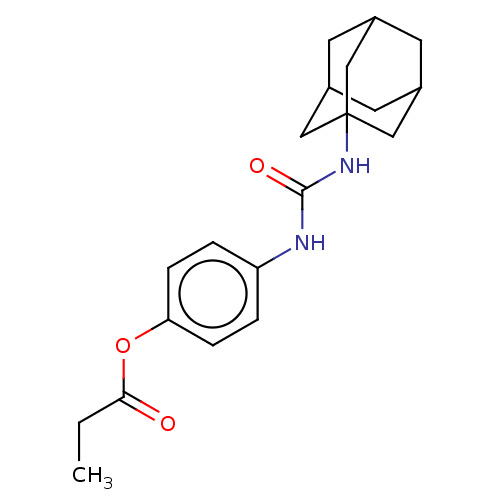

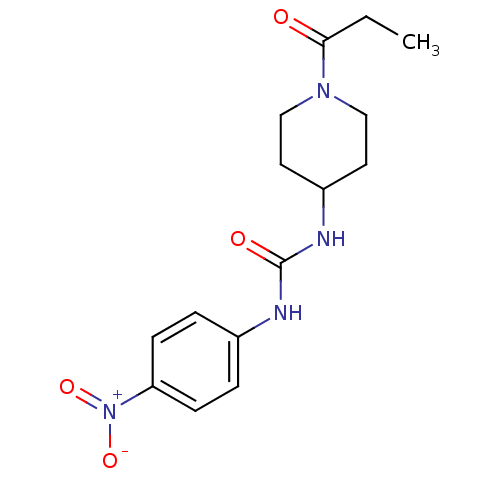
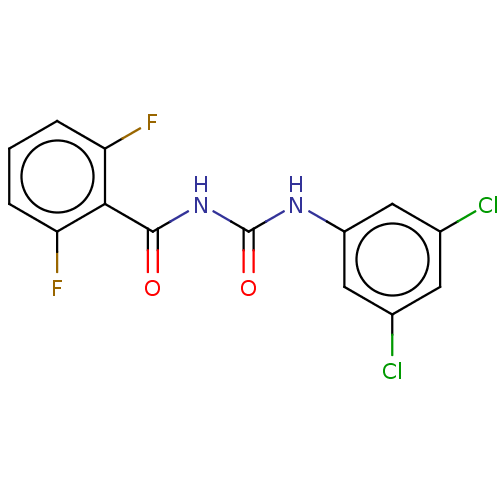
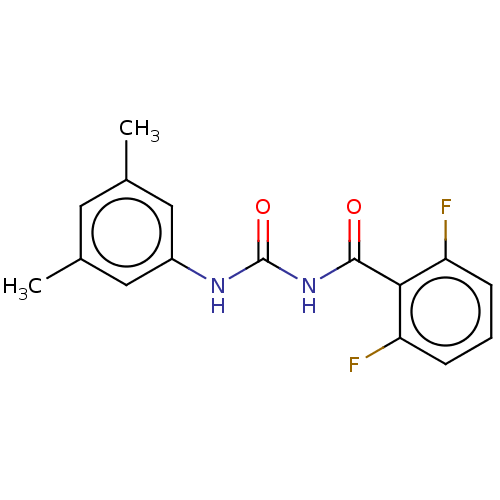
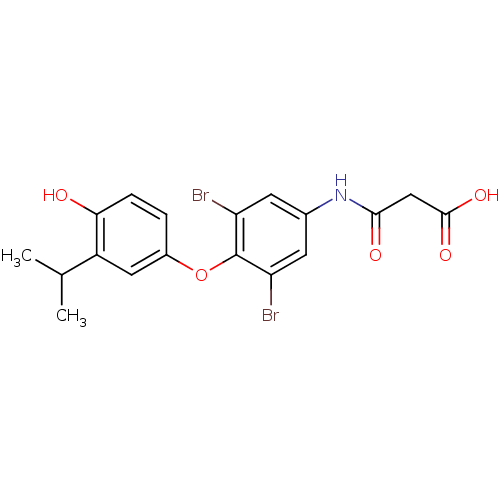
 3D Structure (crystal)
3D Structure (crystal)