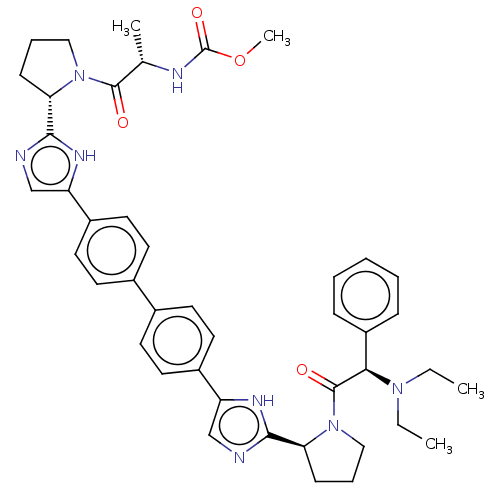
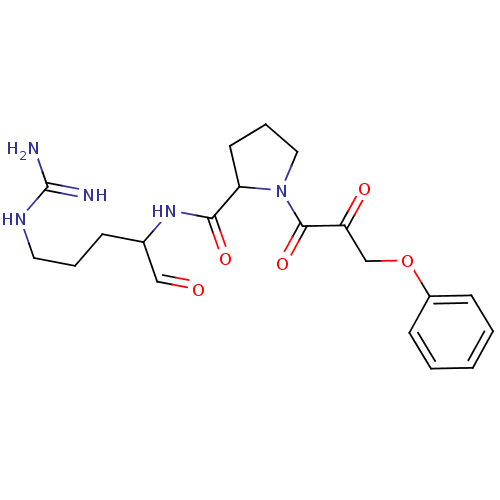
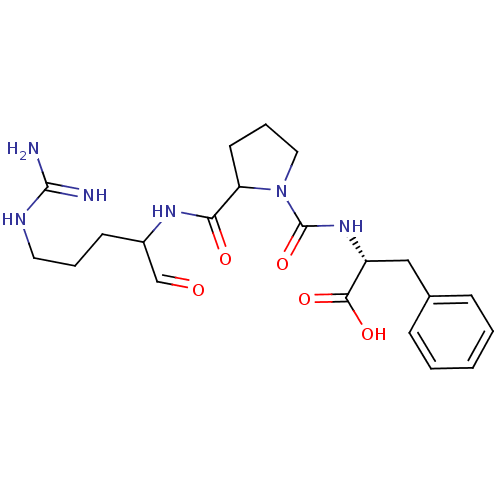
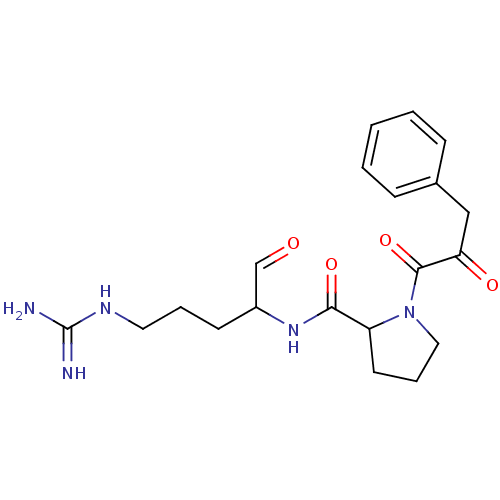
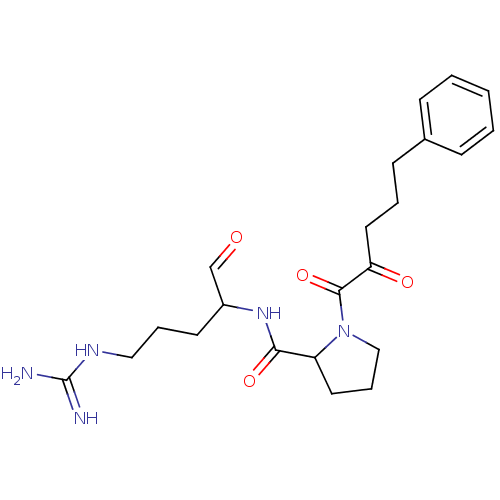
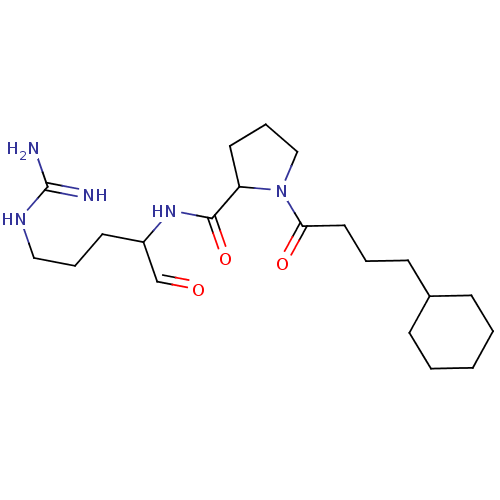
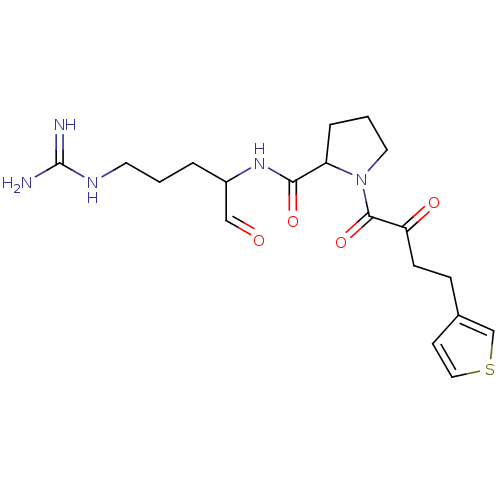
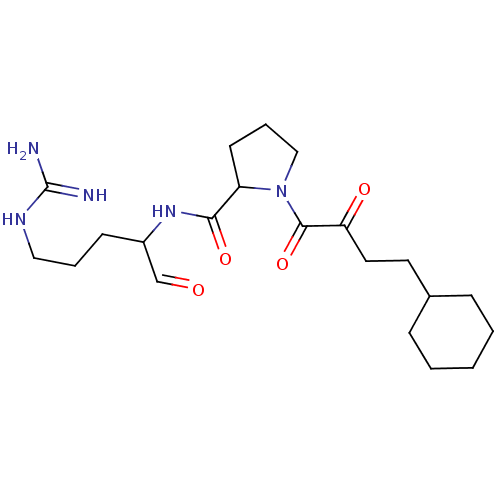
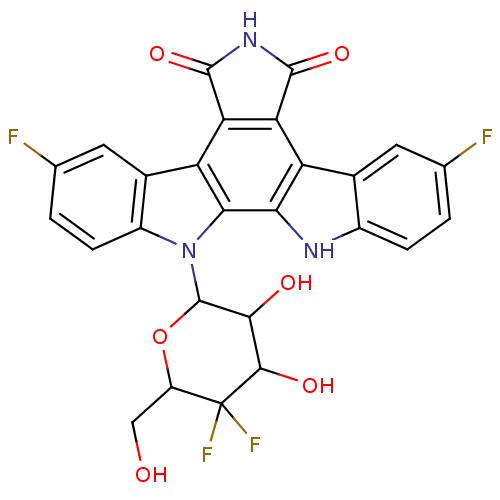
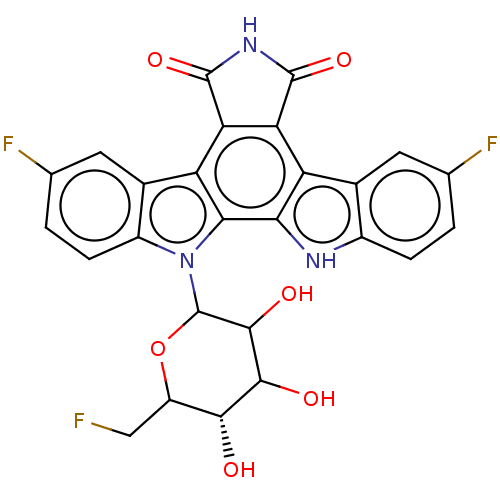
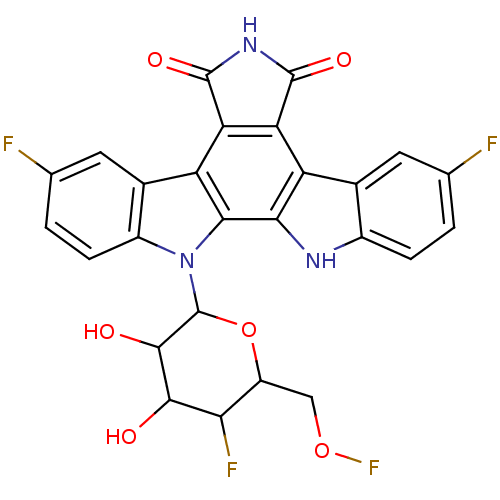
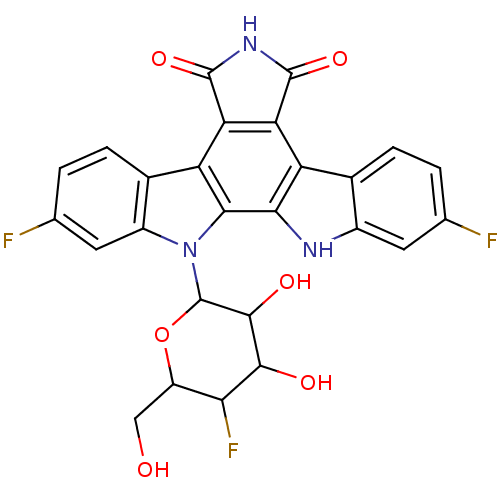
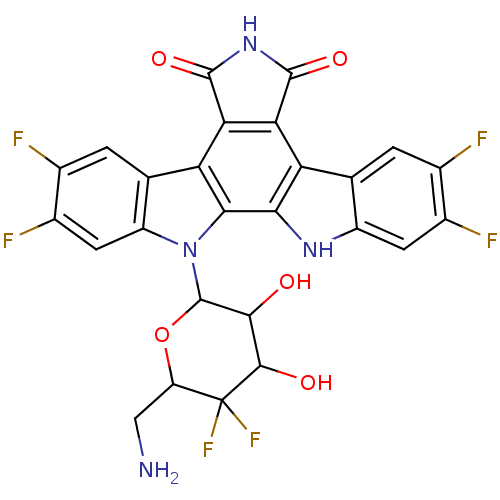
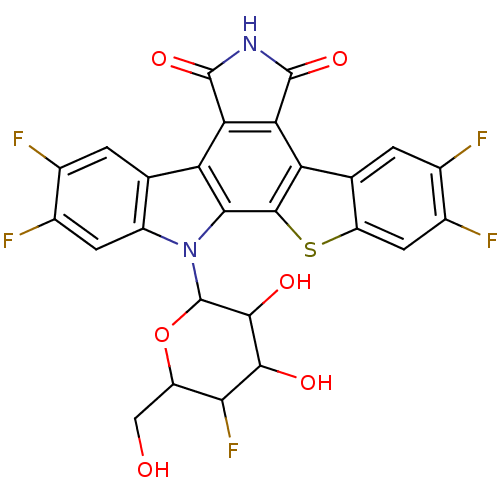
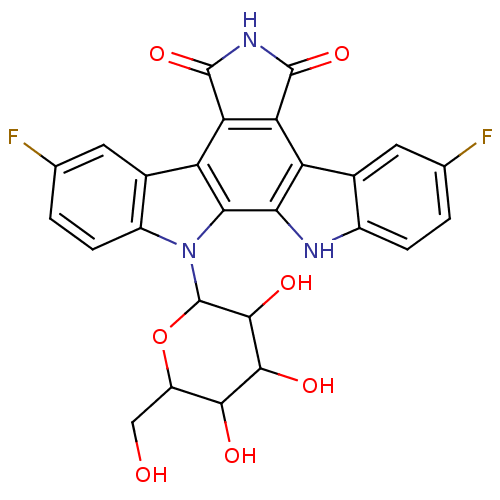
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.10E+3nMAssay Description:Inhibition of human ERG by whole cell patch clamp assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.92E+4nMAssay Description:Inhibition of human ERG by whole cell patch clamp assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 840nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetAnionic trypsin(Bos taurus)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 220nMAssay Description:Inhibitory activity against bovine pancreatic trypsin was determinedMore data for this Ligand-Target Pair
TargetAnionic trypsin(Bos taurus)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 26nMAssay Description:Inhibitory activity against bovine pancreatic trypsin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 590nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.17E+3nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 880nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 2.35E+3nMAssay Description:Inhibitory activity against human plasmin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 5.60E+4nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 590nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 150nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 76.8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 12.8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 240nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 704nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 17.6nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 65.6nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 120nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 32nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 67.2nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 8nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 6.40nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 80nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 480nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 11.2nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetDNA topoisomerase 1(Homo sapiens (Human))
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 38.4nMAssay Description:Topoisomerase I activity for single-strand breaks in the DNA substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 50nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:Inhibitory activity against human thrombin was determinedMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 7.65E+3nMAssay Description:Inhibitory activity against human plasmin was determinedMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 49nMAssay Description:Concentration required to inhibit thrombin hydrolysis of the chromogenic substrateMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:Inhibitory activity against human thrombin was determinedMore data for this Ligand-Target Pair