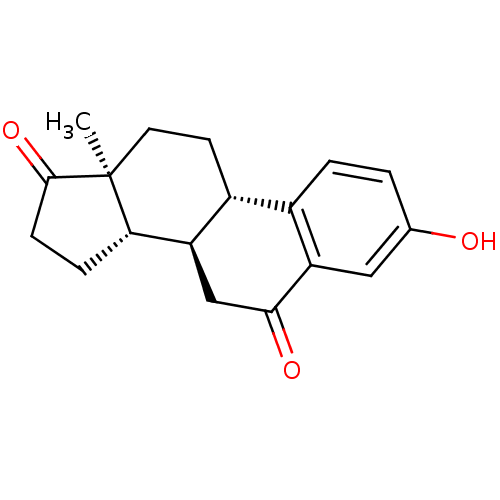
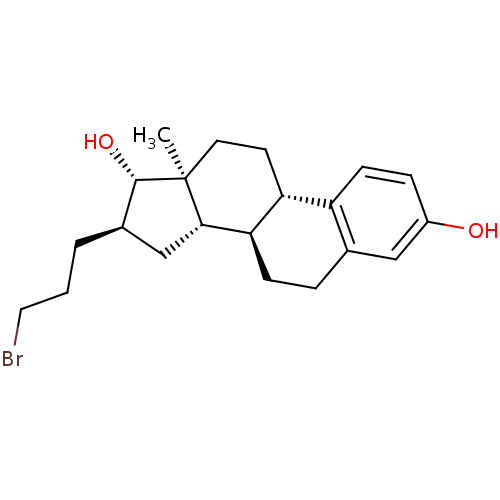
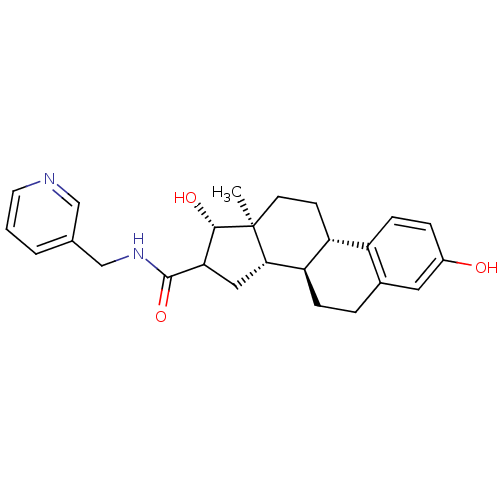
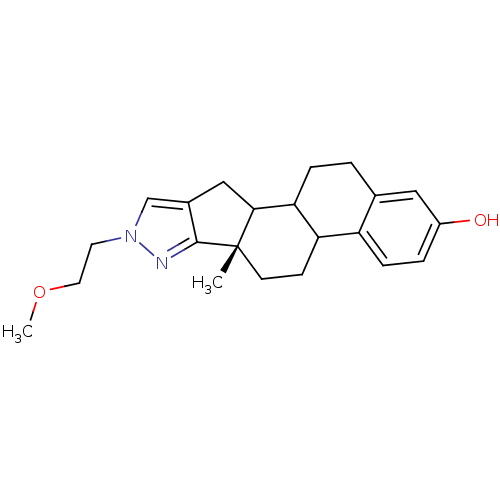
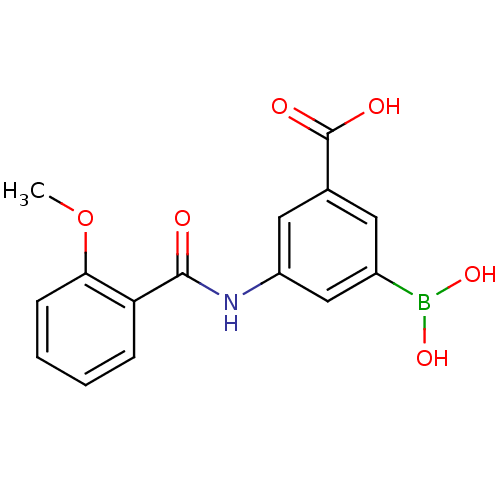
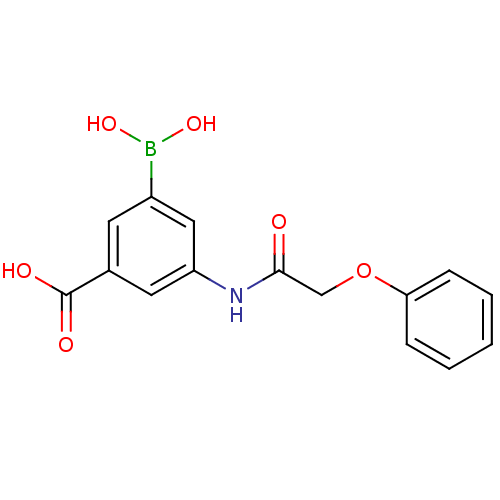
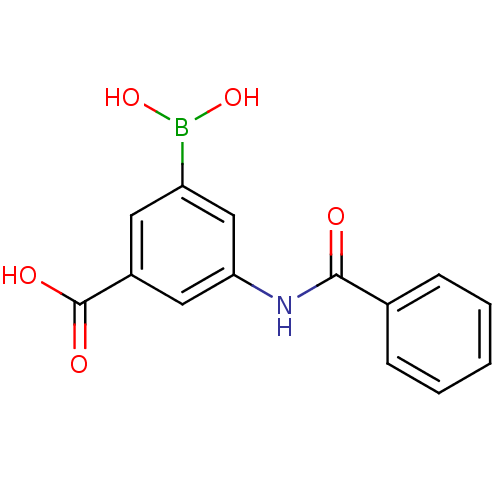
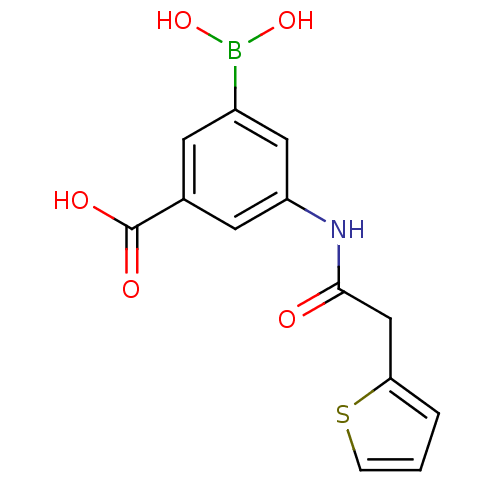
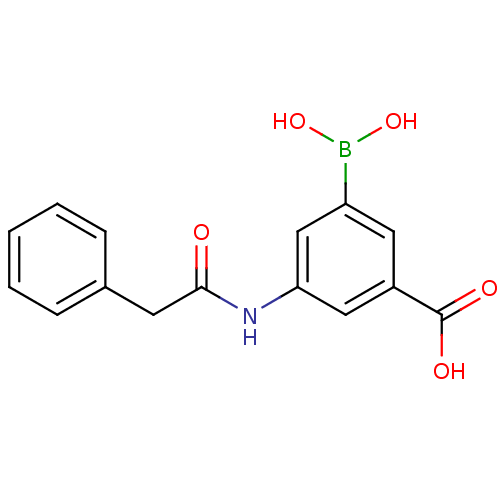
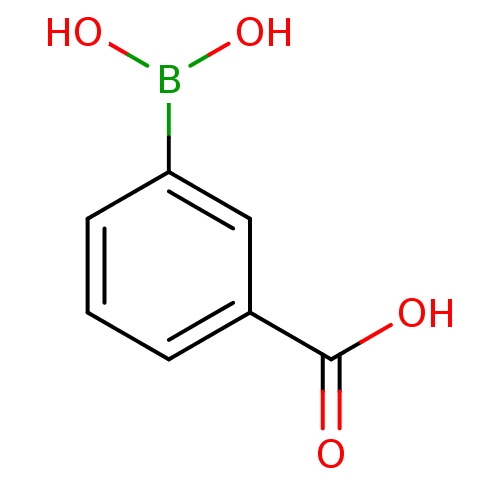
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
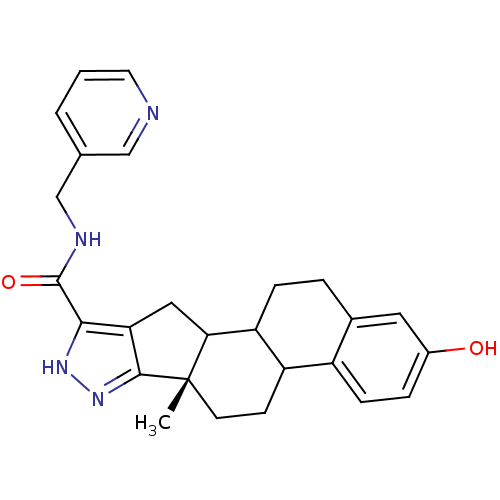
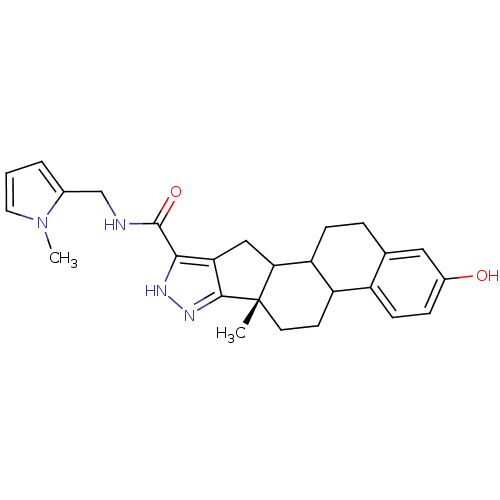
Affinity DataIC50: 37nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
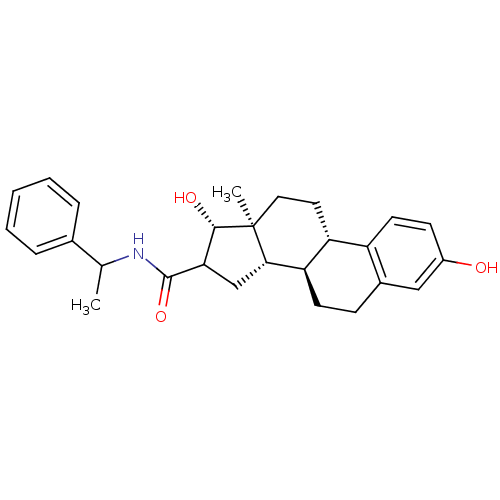
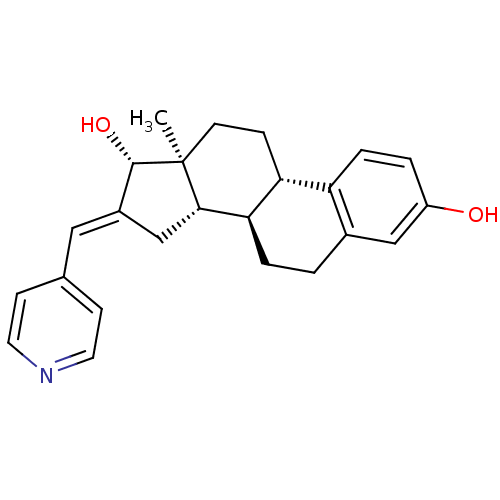
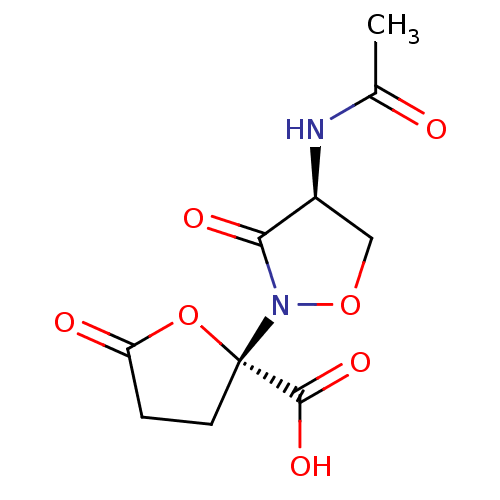
Affinity DataIC50: 110nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
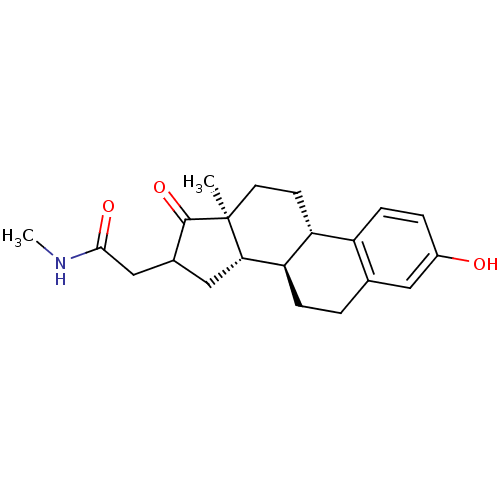
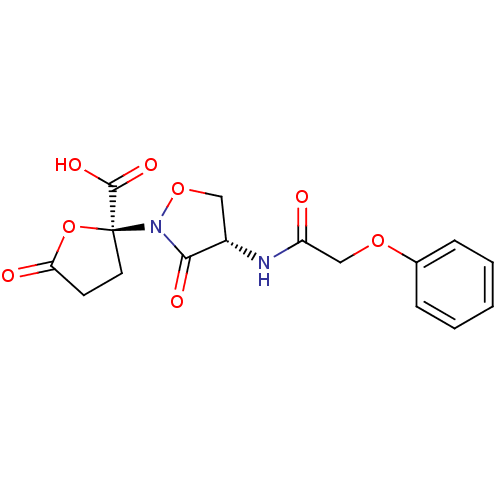
Affinity DataIC50: 110nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
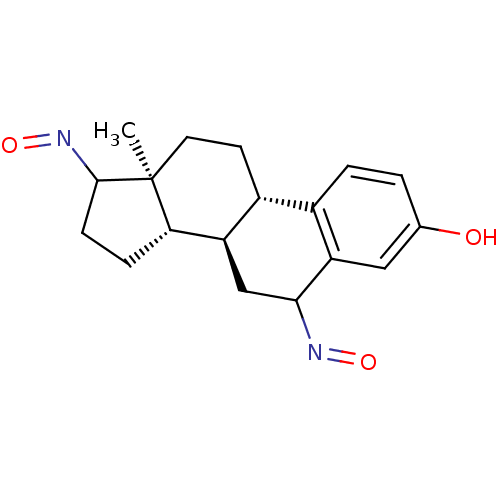
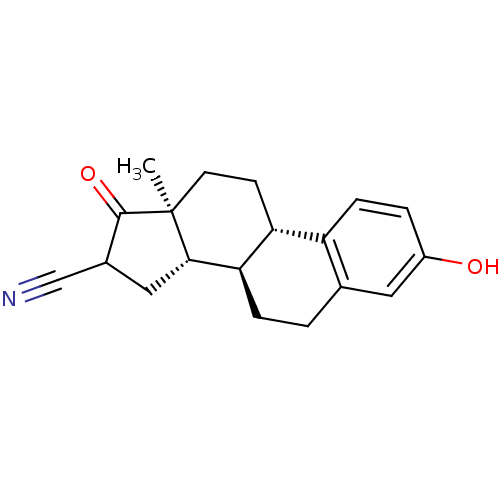
Affinity DataIC50: 180nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 510nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 530nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 780nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 920nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 2.75E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
TargetPenicillin-binding protein 2x(Streptococcus pneumoniae)
Universit£ Joseph Fourier
Curated by ChEMBL
Universit£ Joseph Fourier
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2x after 60 mins by SDS-PAGEMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 7.80E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 8.80E+4nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetPenicillin-binding protein 2x(Streptococcus pneumoniae)
Universit£ Joseph Fourier
Curated by ChEMBL
Universit£ Joseph Fourier
Curated by ChEMBL
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2x after 120 mins by SDS-PAGEMore data for this Ligand-Target Pair
TargetD-alanyl-D-alanine carboxypeptidase(Actinomadura sp. (strain R39))
University of Oxford
Curated by ChEMBL
University of Oxford
Curated by ChEMBL
Affinity DataIC50: 4.00E+5nMAssay Description:Inhibition of Actinomadura sp. R39 penicillin-binding protein preincubated for 60 mins before addition of substrate mixture of (R)-[2-(benzoylamino)p...More data for this Ligand-Target Pair
TargetPenicillin-binding protein 2x(Streptococcus pneumoniae)
Universit£ Joseph Fourier
Curated by ChEMBL
Universit£ Joseph Fourier
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of penicillin-resistant Streptococcus pneumoniae 5204 PBP2X preincubated for 4 hrs before addition of substrate mixture of (R)-[2-(benzoyl...More data for this Ligand-Target Pair


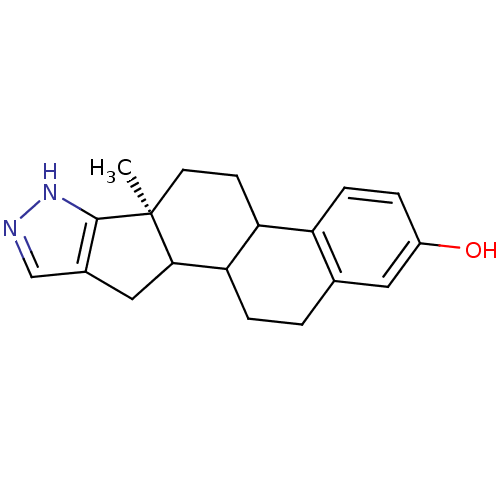

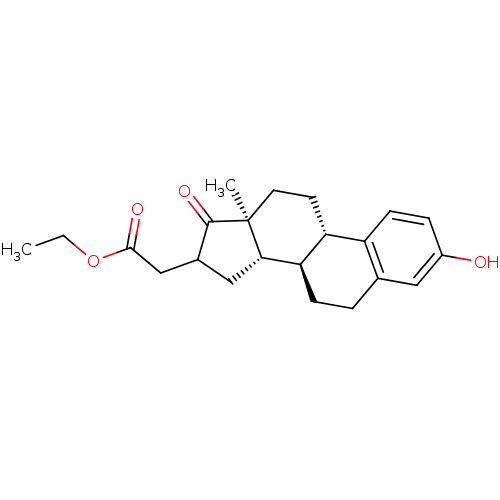
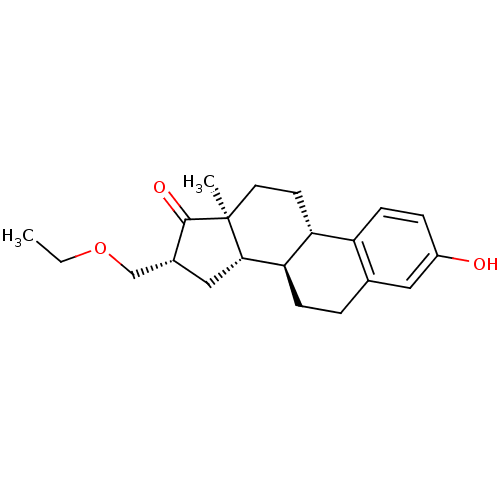

 3D Structure (crystal)
3D Structure (crystal)