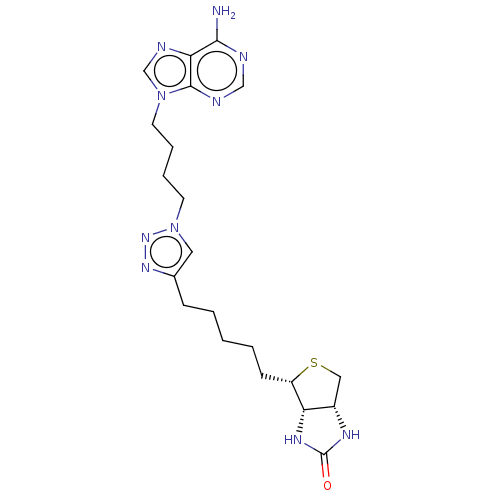
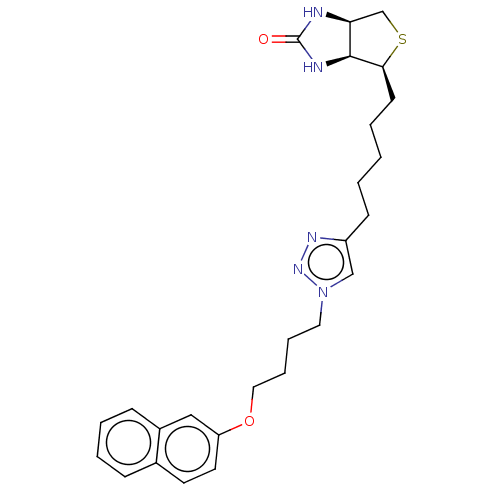
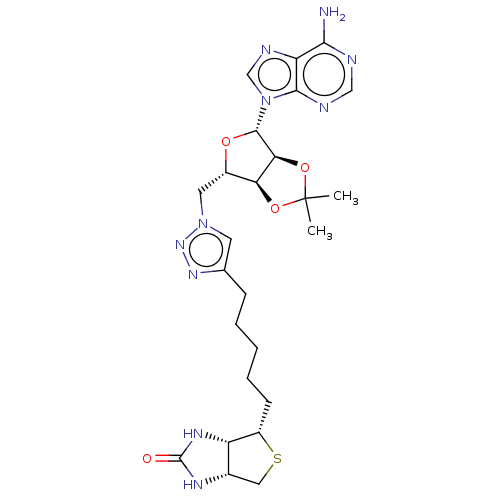
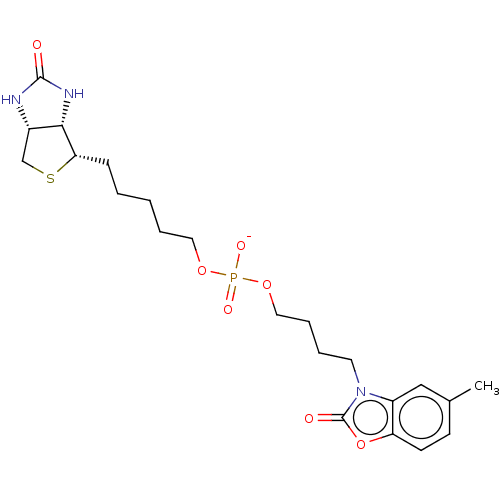
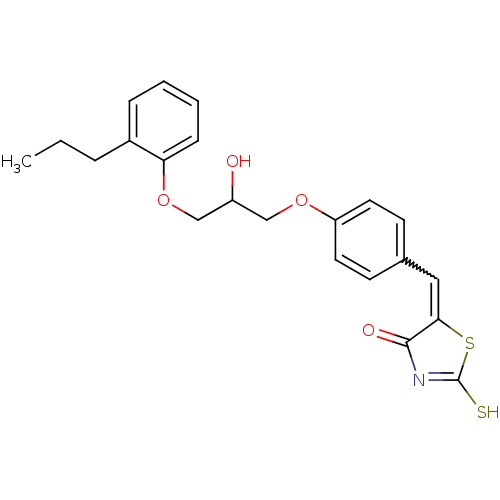
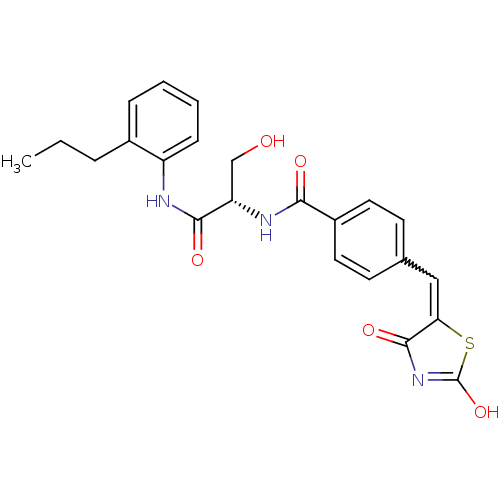
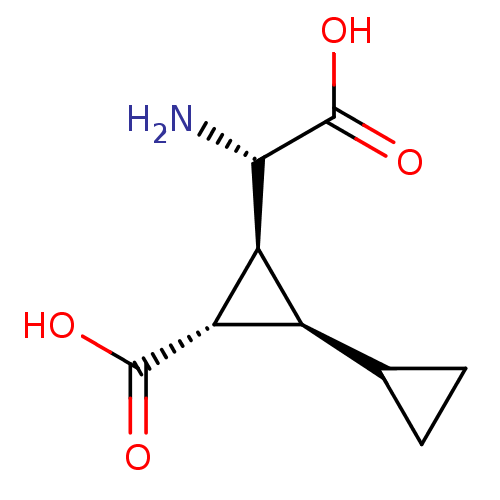
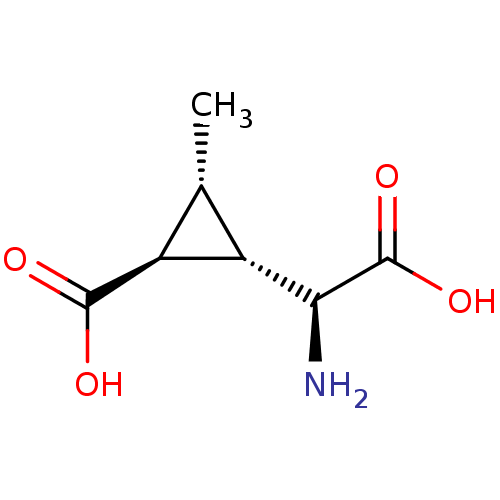
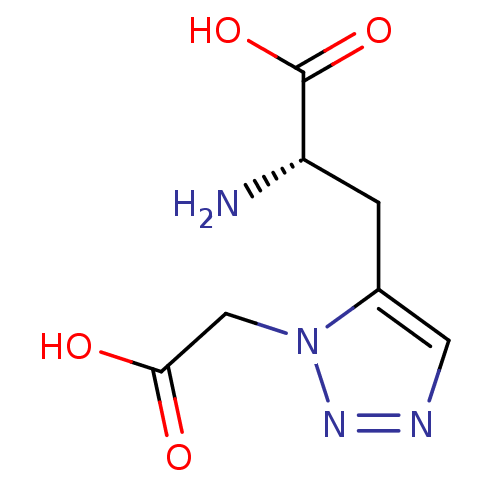
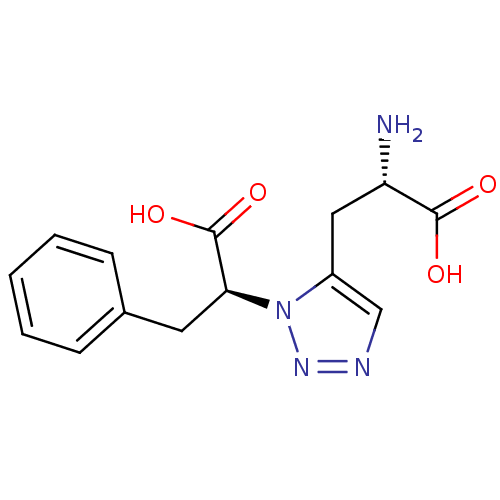
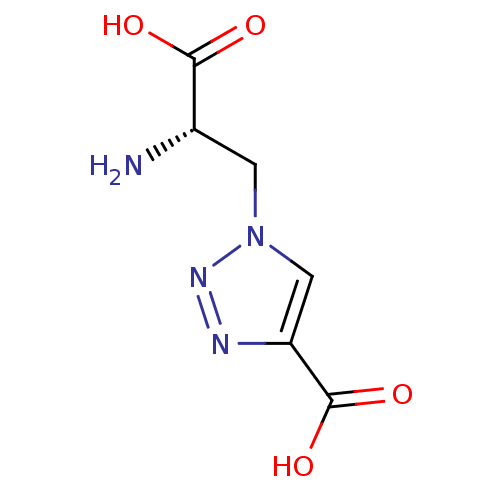
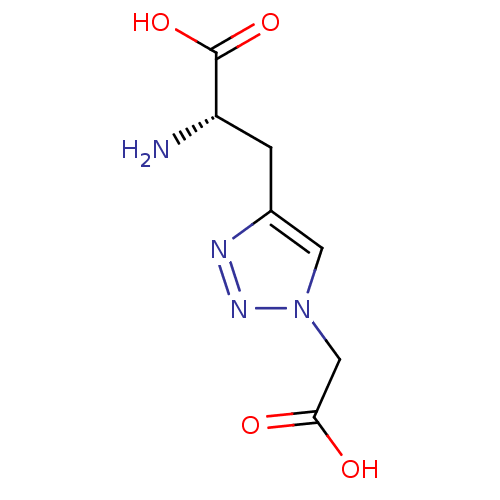
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 30nM ΔG°: -44.7kJ/mole IC50: 203nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
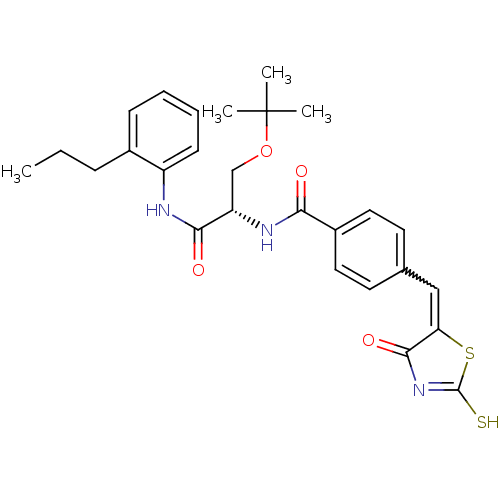
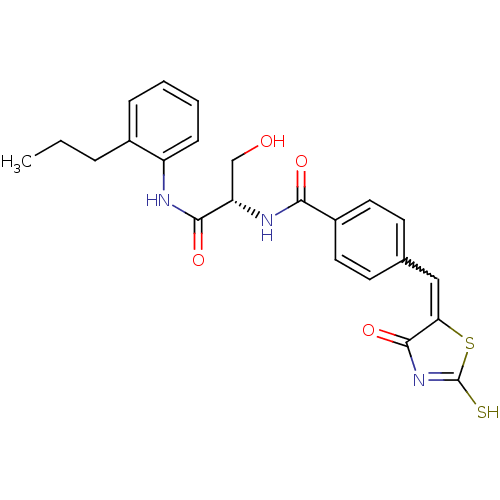
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 90nM ΔG°: -41.8kJ/mole IC50: 530nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
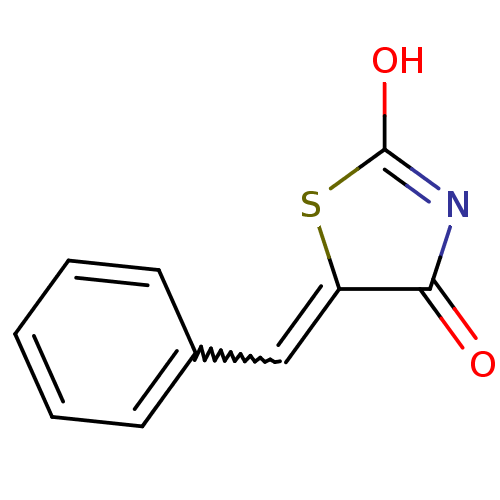
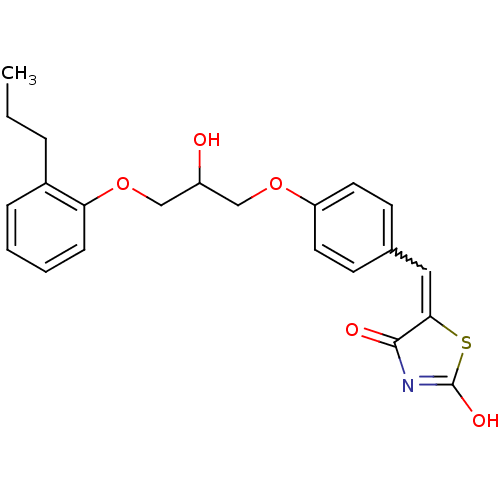
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 225nM ΔG°: -39.5kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
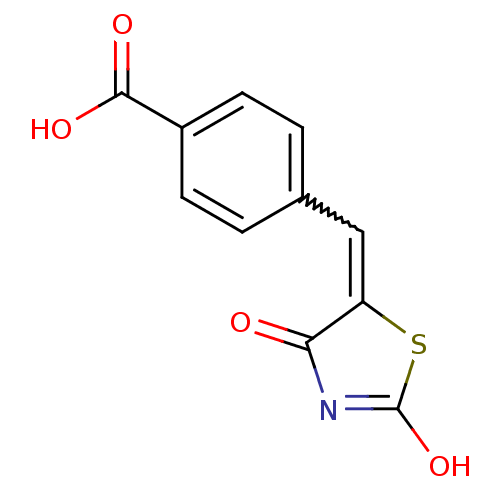
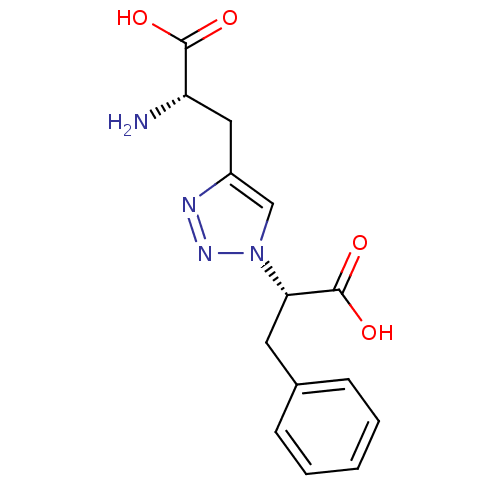
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 420nM ΔG°: -37.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 660nM ΔG°: -36.7kJ/mole IC50: 4.00E+3nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 1.17E+3nM ΔG°: -35.2kJ/mole IC50: 7.00E+3nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: 1.83E+3nM ΔG°: -34.1kJ/mole IC50: 1.16E+4nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Staphylococcus aureus)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >1.00E+4nM ΔG°: >-29.7kJ/mole IC50: 5.00E+4nMpH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetBifunctional ligase/repressor BirA(Escherichia coli)
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Monash University; Adelaide Research & Innovation Pty Ltd
US Patent
Affinity DataKi: >3.00E+4nM ΔG°: >-26.9kJ/molepH: 8.0 T: 2°CAssay Description:Quantitation of BPL catalysed 3H-biotin incorporation into the biotin domain substrate was performed as previously described by Polyak et al, J. Biol...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 5.14E+3nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 2.16E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 5.70E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 6.20E+4nMAssay Description:Antagonist activity at mGlu2 receptor expressed in CHO cells assessed as increase of cAMP levelMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 6.60E+4nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Electrophorus electricus acetylcholinesterase preincubated for 15 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP levelMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP levelMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation countingMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu4 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP levelMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >50nMAssay Description:Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP levelMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >10nMAssay Description:Agonist activity at mGlu2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP levelMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation countingMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Agonist activity at mGlu1 receptor expressed in CHO cells assessed as myo-[2-3H]Inositol turnover by scintillation countingMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR4 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 2(Homo sapiens (Human))
The University of Adelaide
Curated by ChEMBL
The University of Adelaide
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Binding affinity at mGluR2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 10 minsMore data for this Ligand-Target Pair

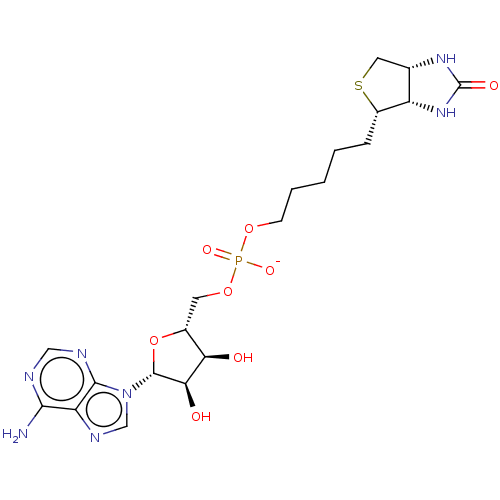
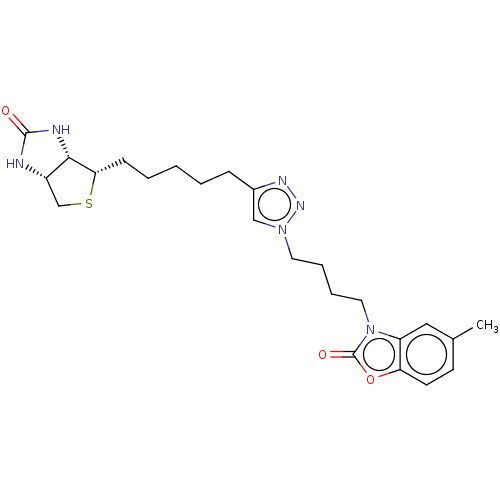
 3D Structure (crystal)
3D Structure (crystal)