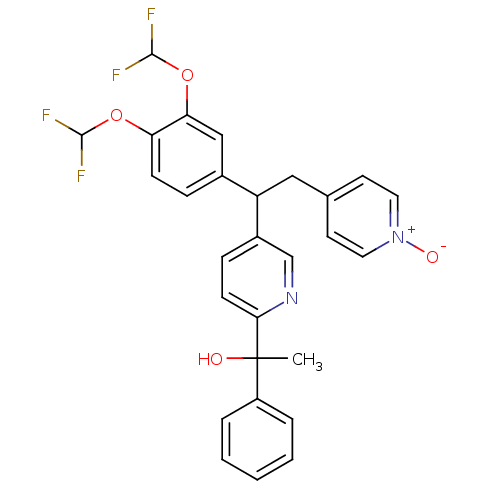
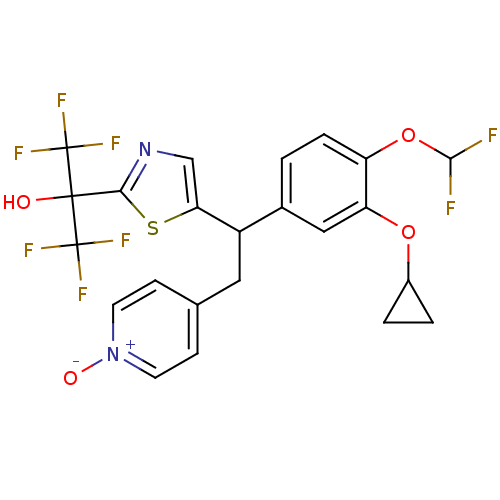
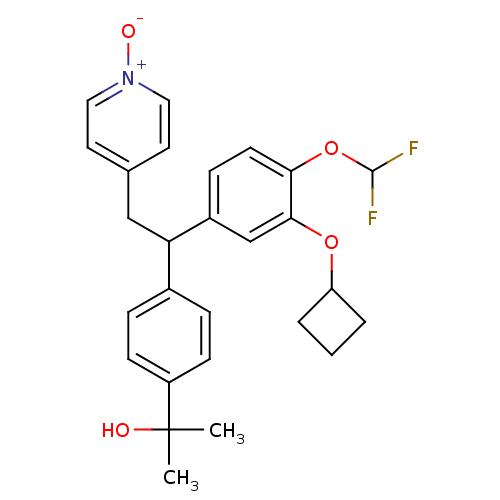
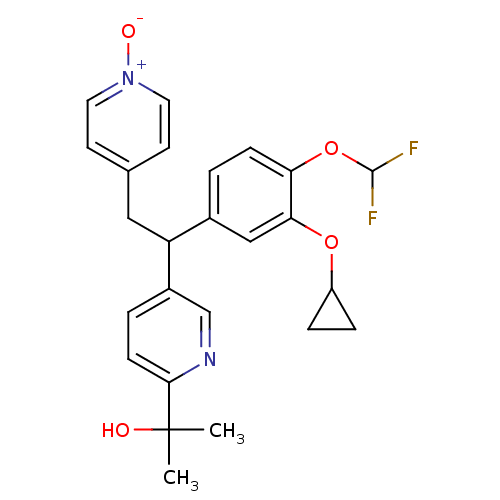
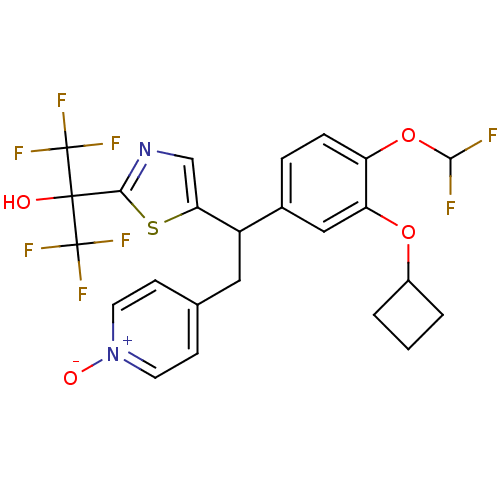
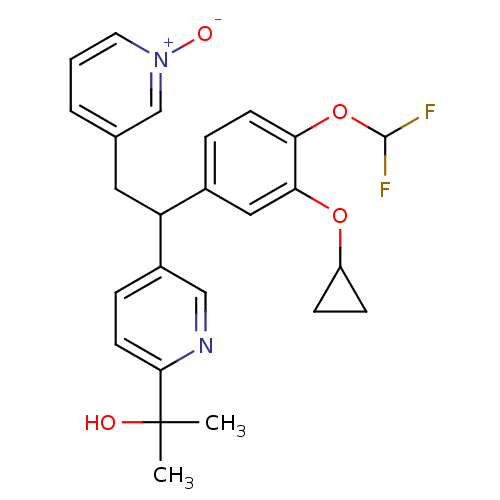
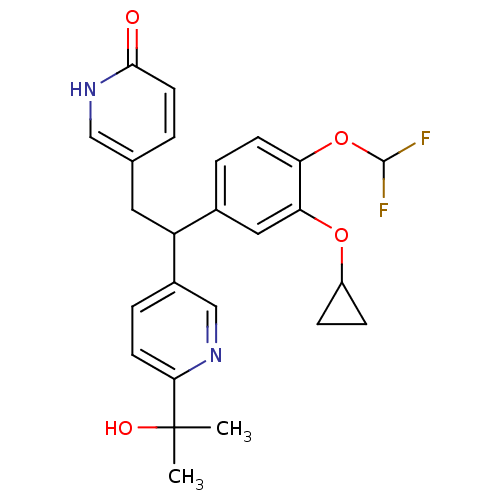
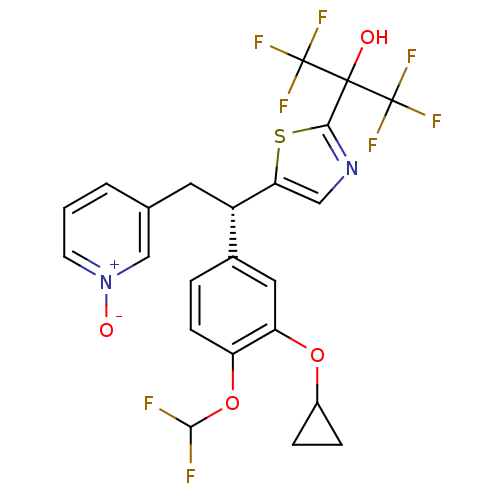
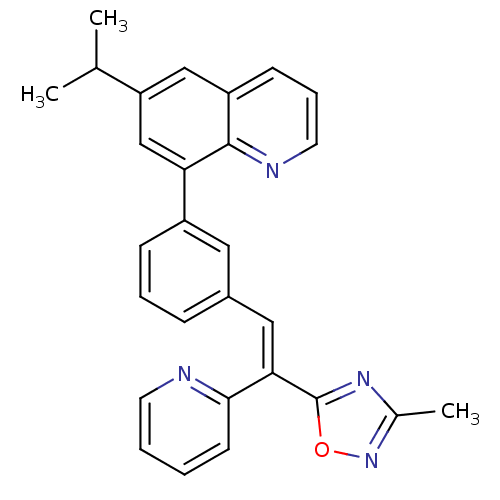
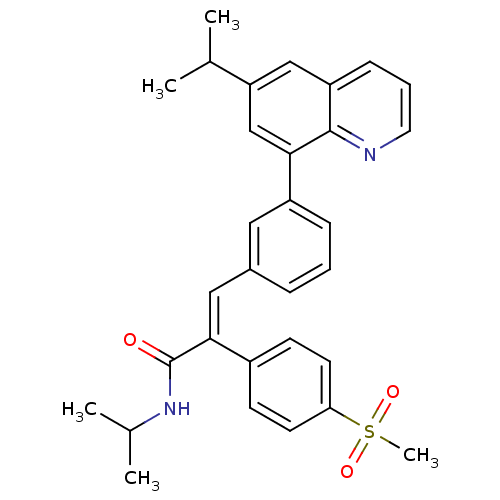
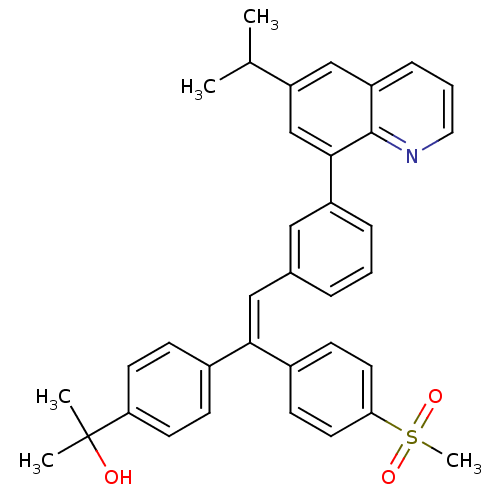
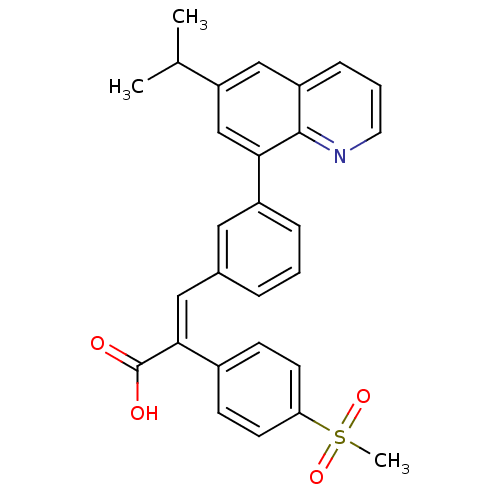
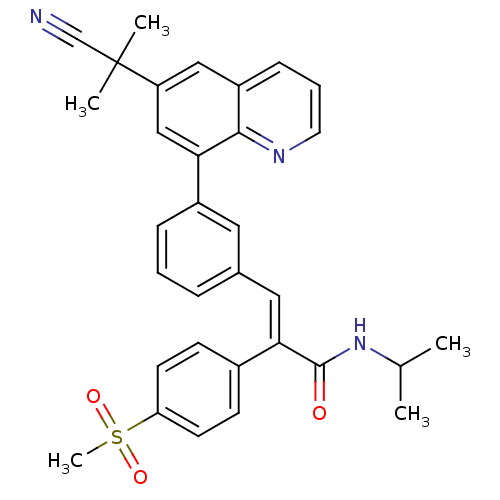
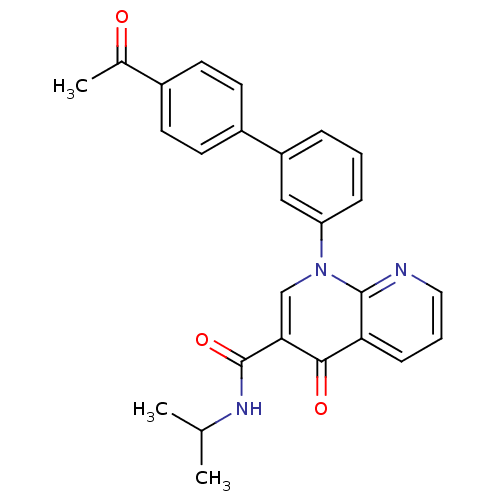
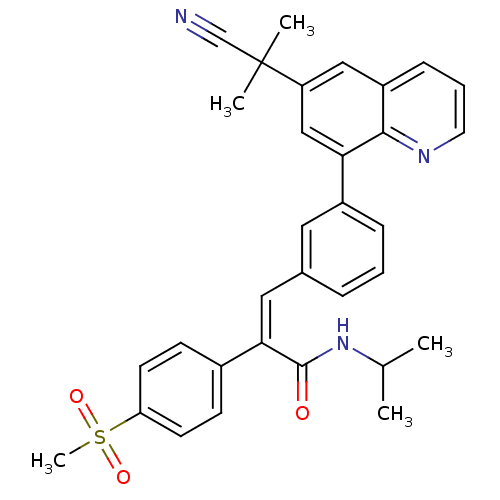
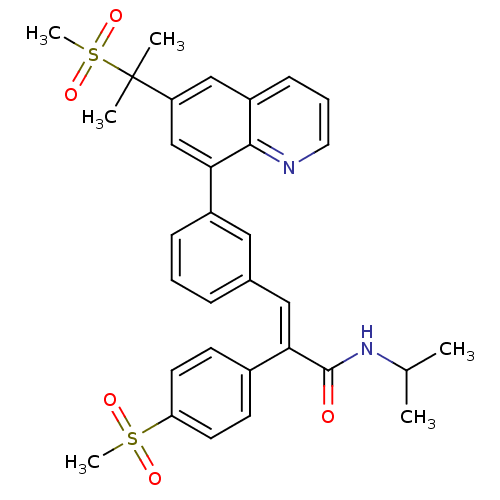
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 1.18E+3nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.10E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.24E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.27E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.27E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.50E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.64E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.64E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 3.81E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 3.90E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 4.22E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 4.47E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 5.59E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 5.84E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 6.14E+4nMAssay Description:In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.0700nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.170nMAssay Description:Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.190nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.370nMAssay Description:Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4C(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4CMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.450nMAssay Description:Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cellsMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4C(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4CMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against PDE4DMore data for this Ligand-Target Pair