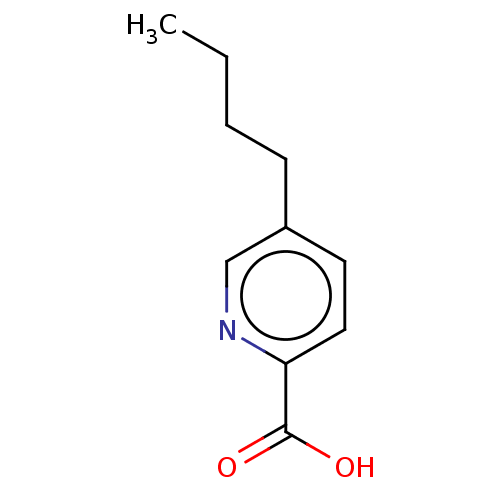
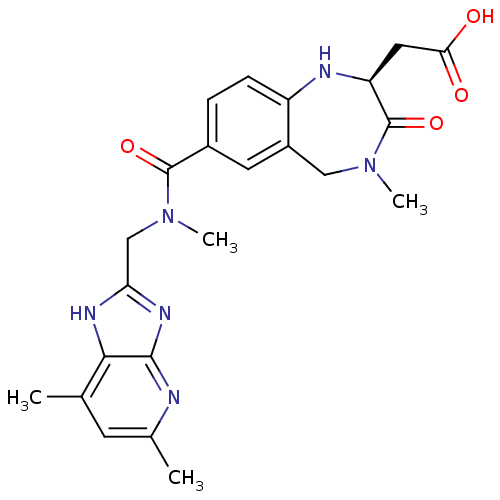
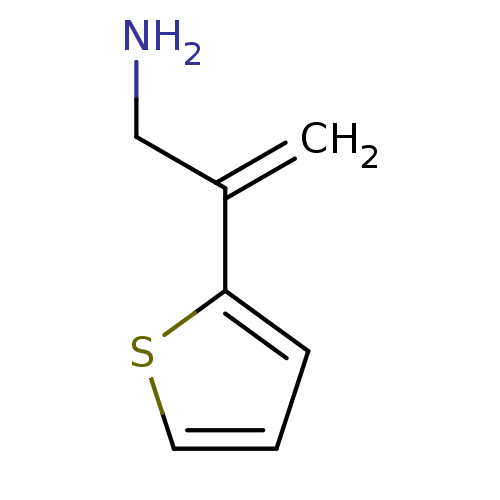
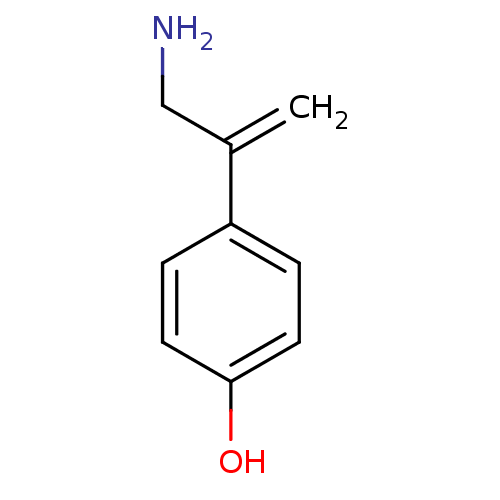
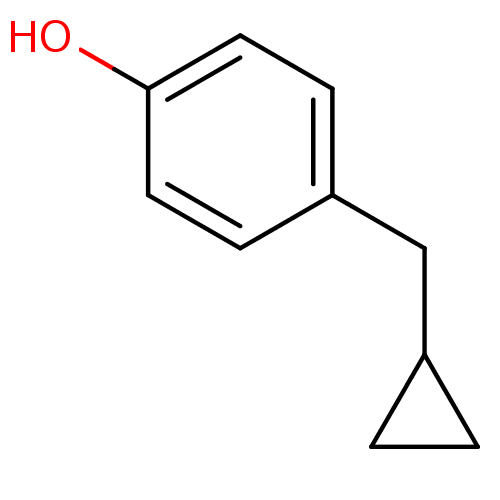
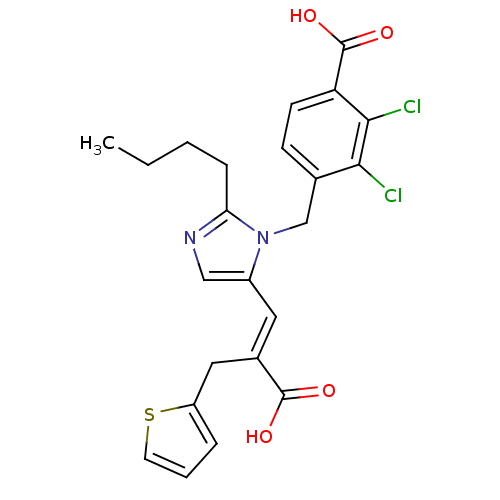
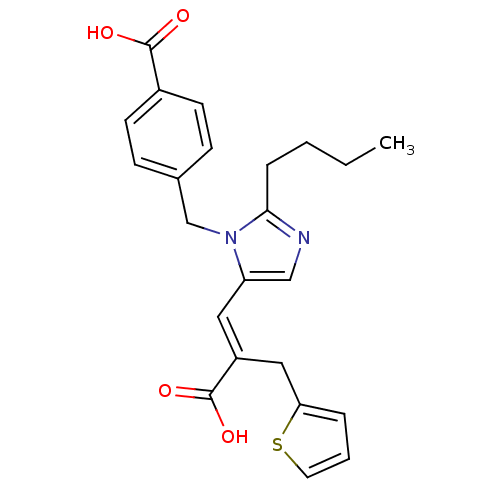
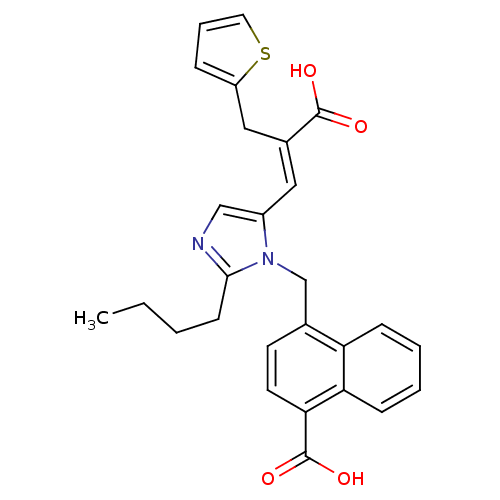
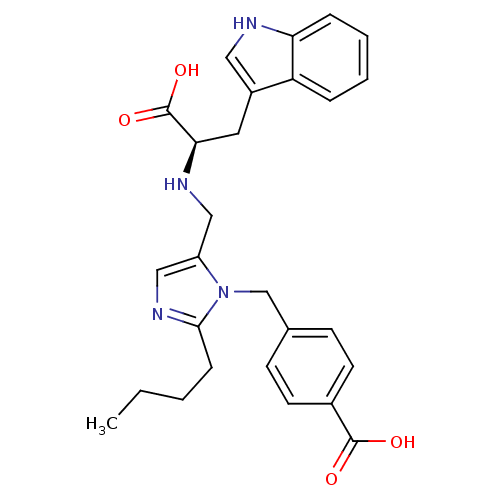
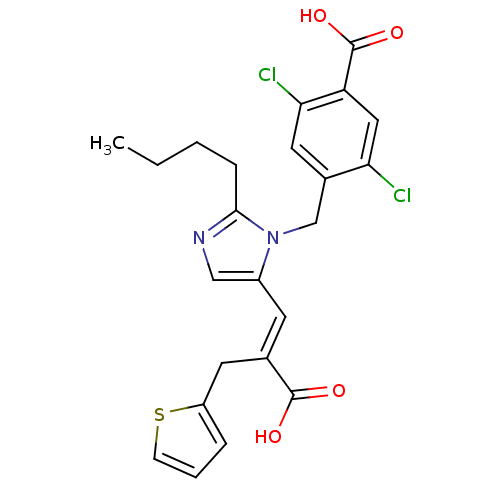
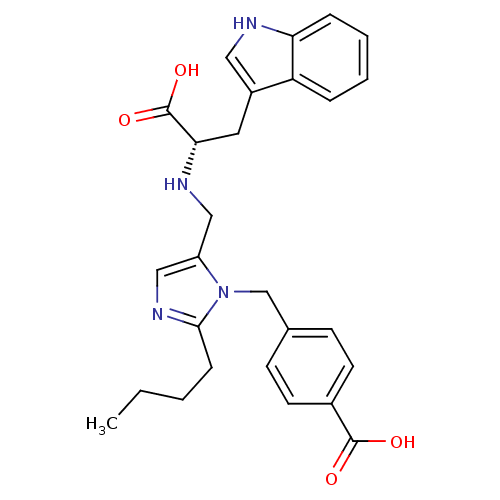
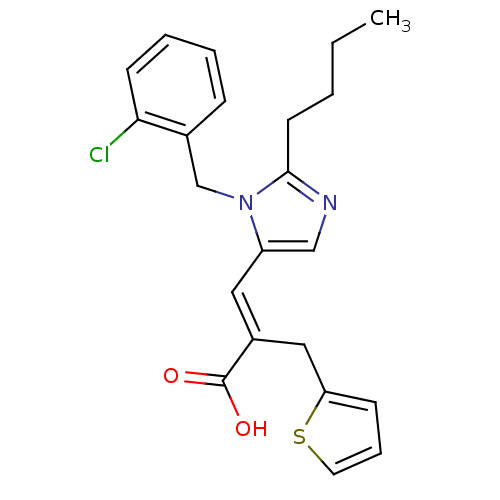
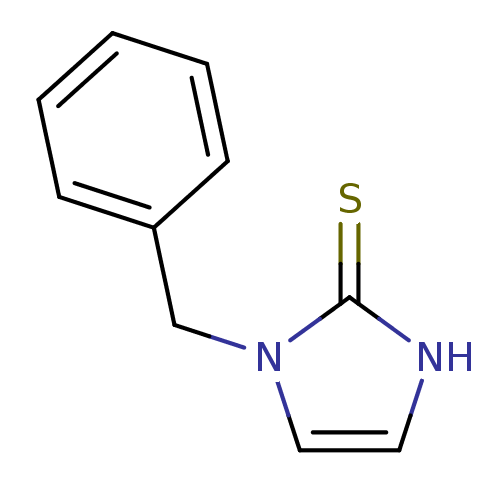
TargetEndothelin-1 receptor(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin-1 receptor(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin-1 receptor(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMpH: 4.5Assay Description:Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarateMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 23nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
Affinity DataKi: 41nMpH: 4.5Assay Description:Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarateMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 45nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
Affinity DataKi: 55nMpH: 4.5Assay Description:Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarateMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
Affinity DataKi: 149nMpH: 4.5Assay Description:Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarateMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetEndothelin receptor type B(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
TargetIntegrin alpha-V/beta-3(Homo sapiens (Human))
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 710nMAssay Description:Antagonistic activity for human vitronectin receptor (alphaV-beta3) from plateletsMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+4nMAssay Description:KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]More data for this Ligand-Target Pair
Affinity DataKi: 5.70E+4nMAssay Description:KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]More data for this Ligand-Target Pair
Affinity DataKi: 5.70E+4nMAssay Description:KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+5nMpH: 5.0Assay Description:KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]; Apparent values at pH 5.0, 0.24 mM O2More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+6nMAssay Description:KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the absence of BSAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the absence of bovine serum albumin (BSA)More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Tested for inhibition of Angiotensin II specific binding to Angiotensin II receptor, type 1 in the recombinant human AT-1 receptor expressed in LhAT-...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the presence of 0.25% bovine serum albumin (BSA)More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Tested for Angiotensin II receptor, type 1 affinity in the presence of 0.25% BSAMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Tested for inhibition of [125I]- AII specific binding to rat mesenteric arteries, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 48.3nMAssay Description:Tested for inhibition of Angiotensin II specific binding to Angiotensin II receptor, type 1 in the recombinant human AT-1 receptor expressed in LhAT-...More data for this Ligand-Target Pair
Affinity DataIC50: 298nMAssay Description:Tested for inhibition of Angiotensin II specific binding to Angiotensin II receptor, type 1 in the recombinant human AT-1 receptor expressed in LhAT-...More data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Tested for binding affinity to Angiotensin II receptor, type 1More data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 0.4-1.1More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 1.1-1.4More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 1.3-4.6More data for this Ligand-Target Pair
Affinity DataIC50: 4.53E+3nMAssay Description:Tested for binding affinity to Angiotensin II receptor, type 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value range from 8.9-13More data for this Ligand-Target Pair

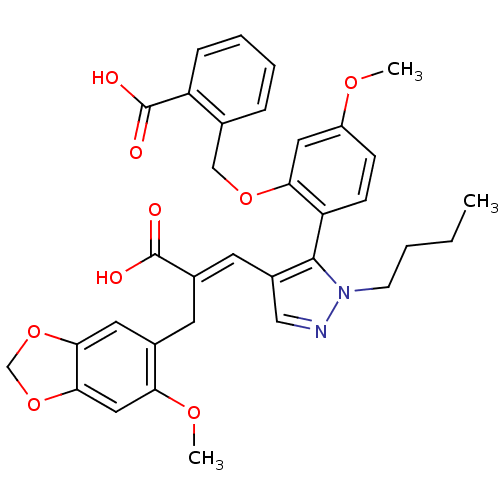
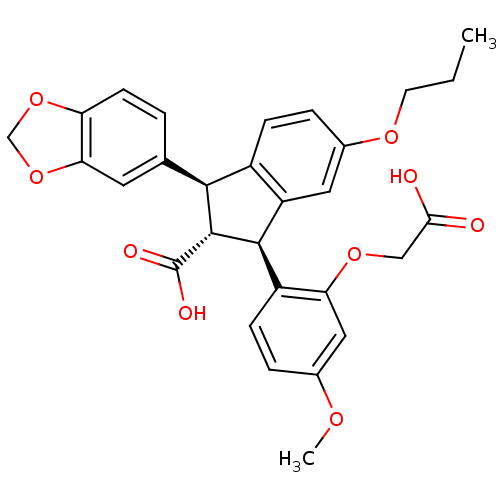
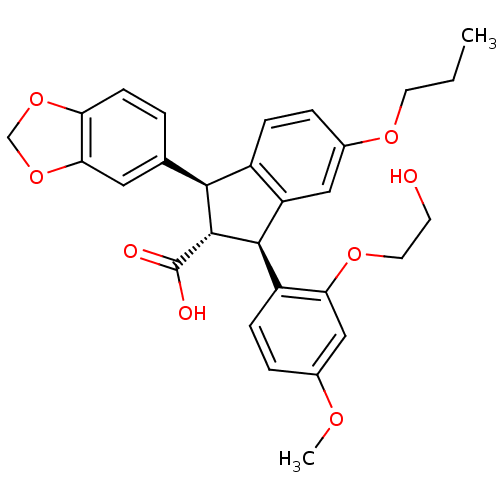
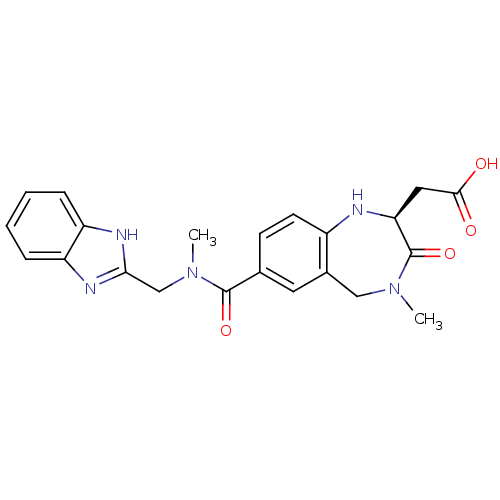
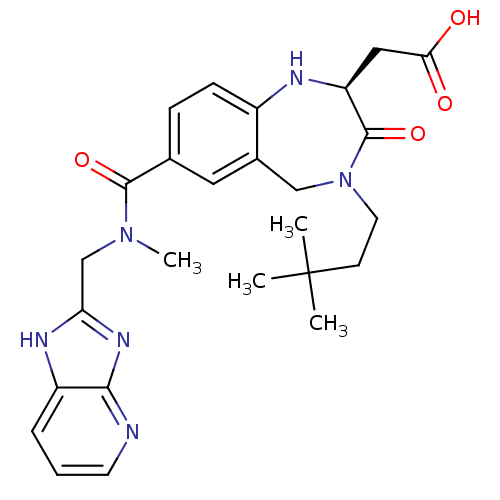

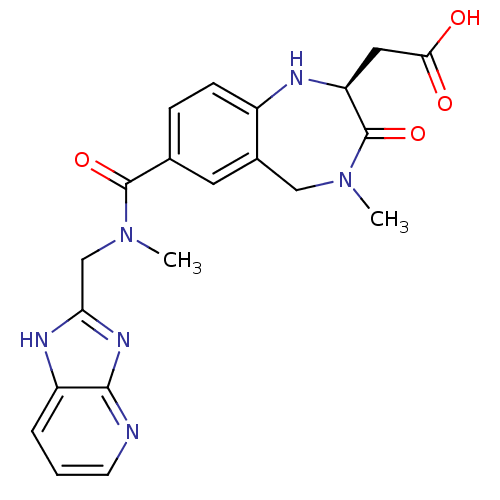


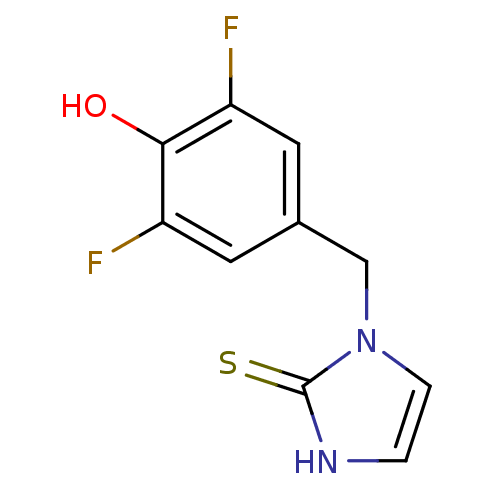
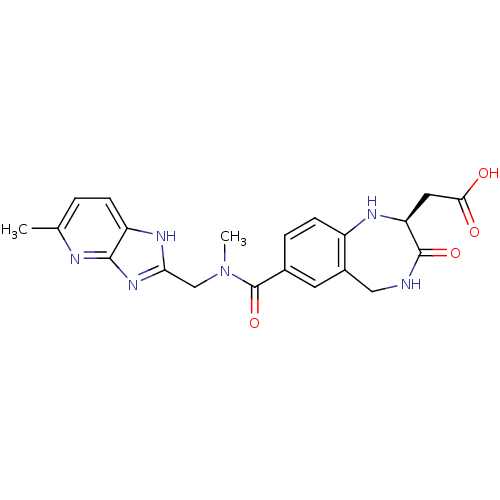
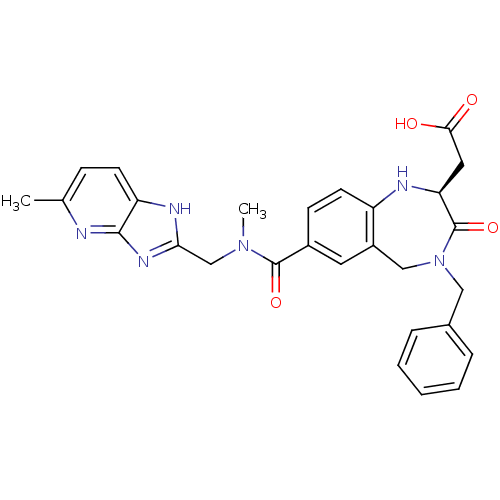
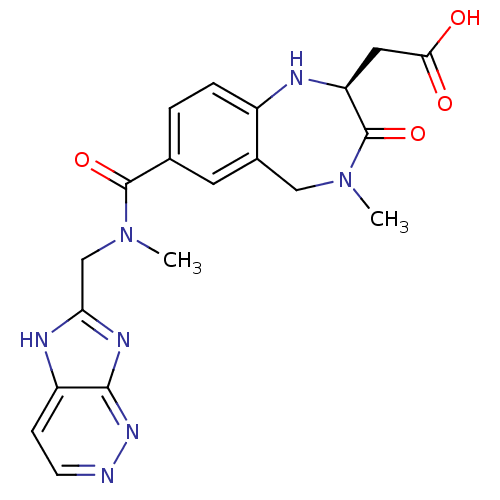
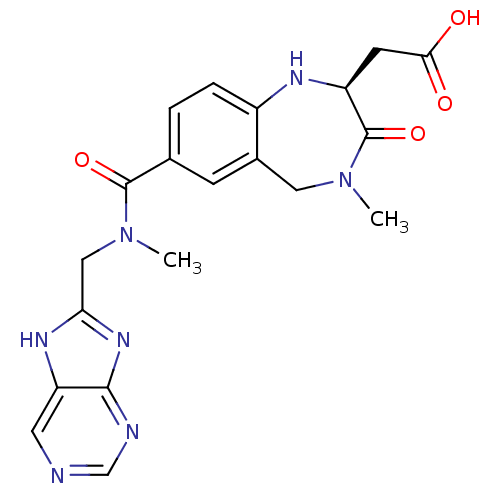
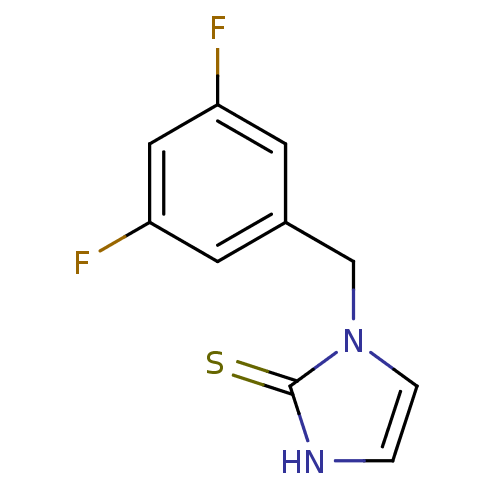
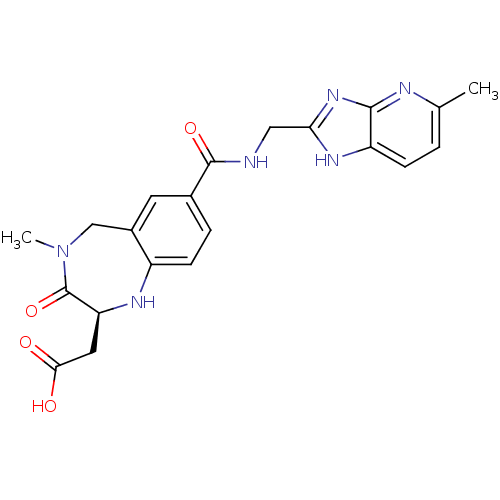

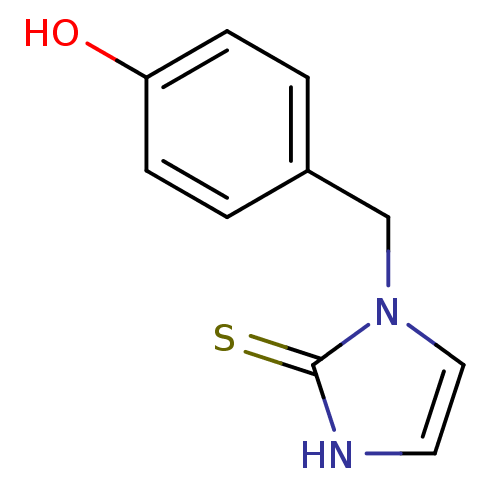
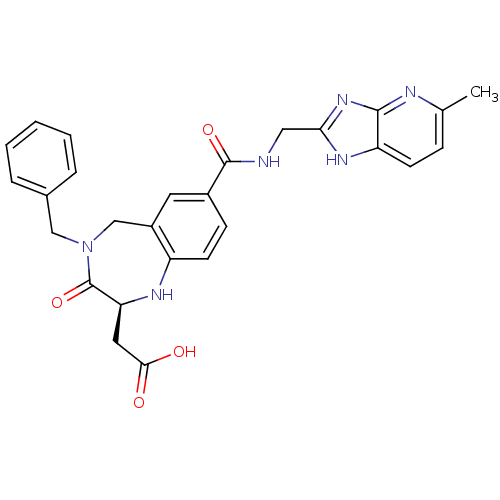
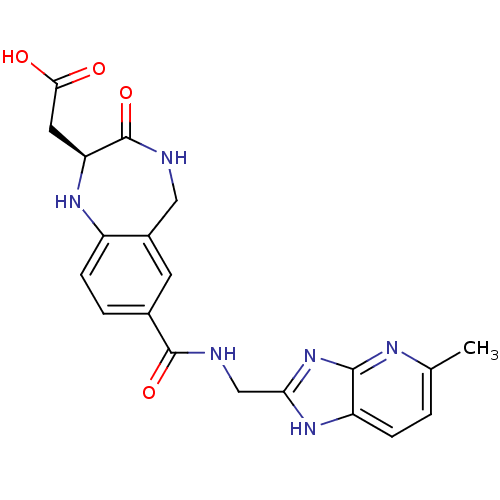
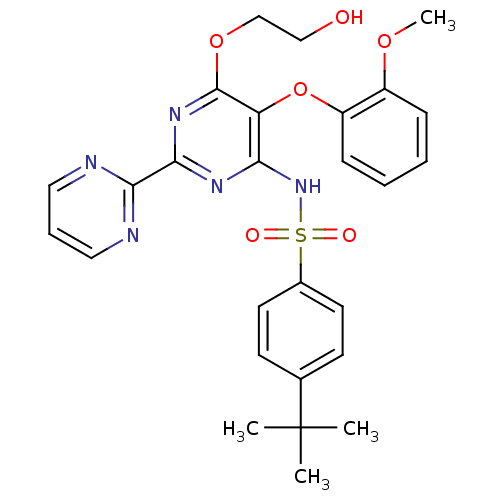
 3D Structure (crystal)
3D Structure (crystal)