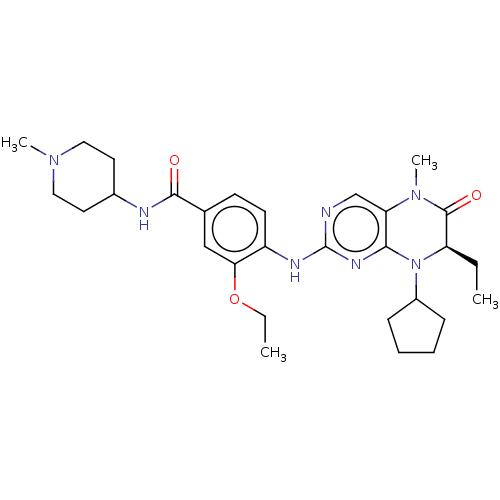
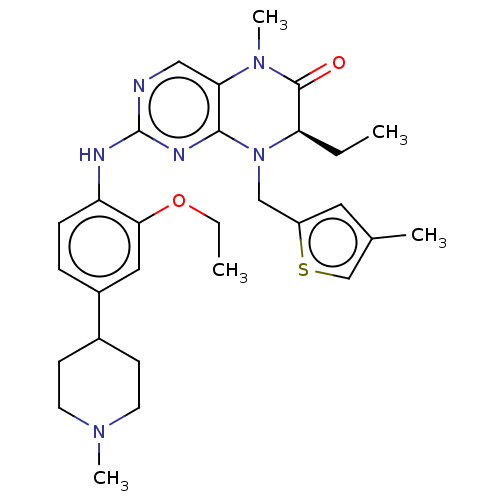
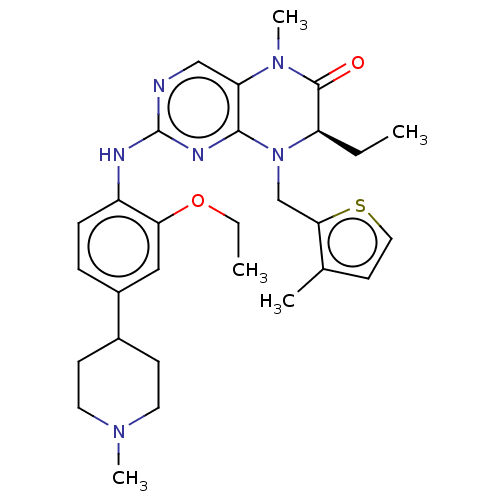
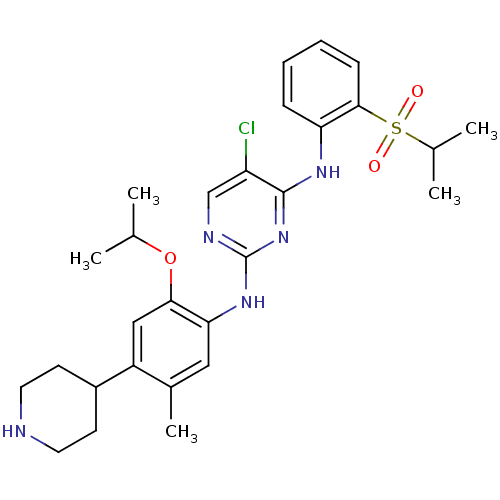
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
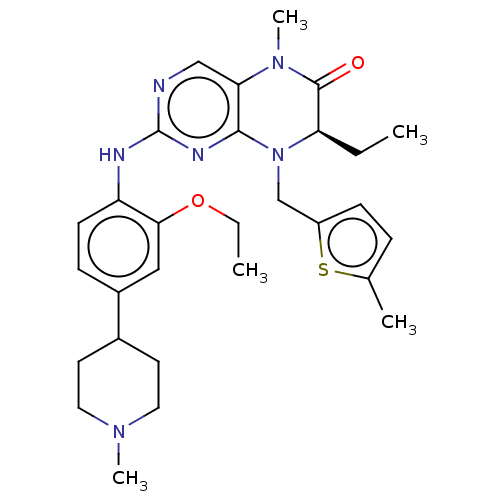
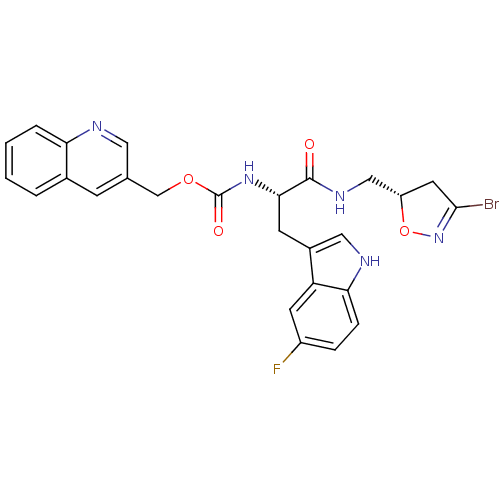
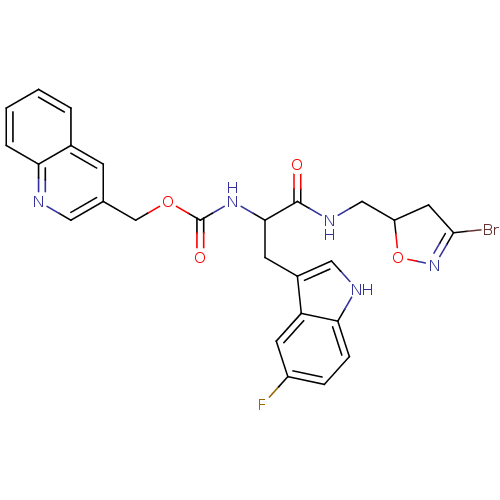
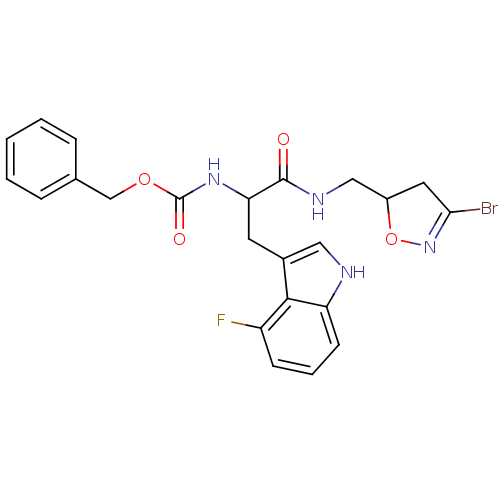
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
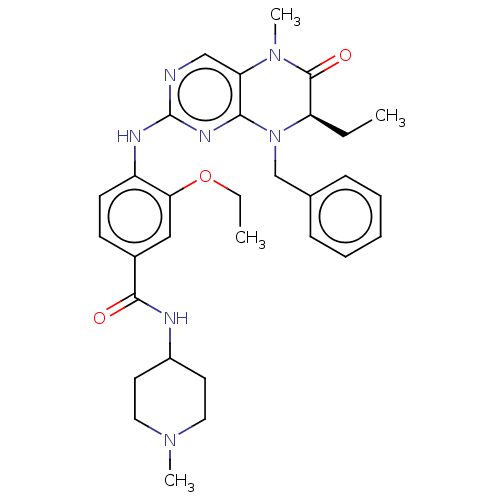
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
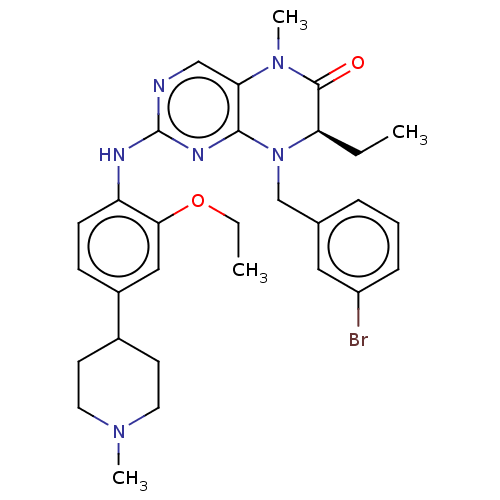
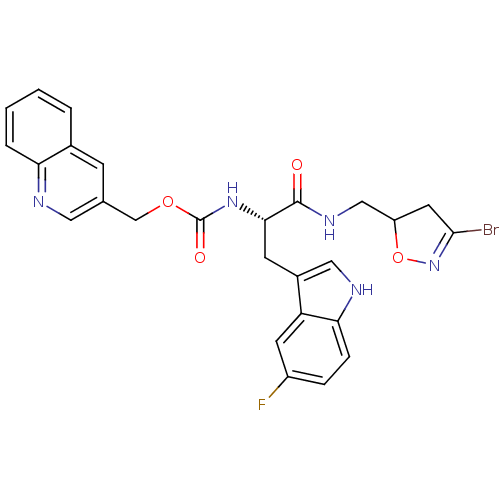
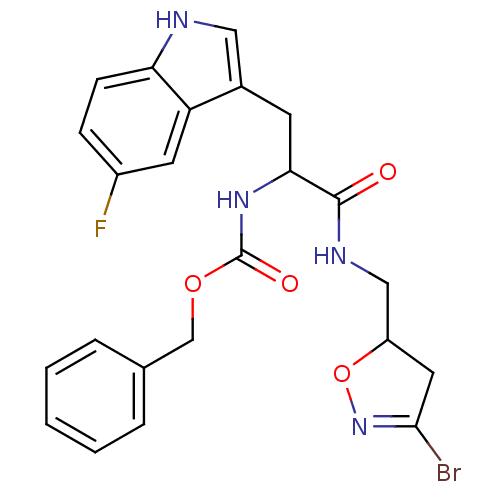
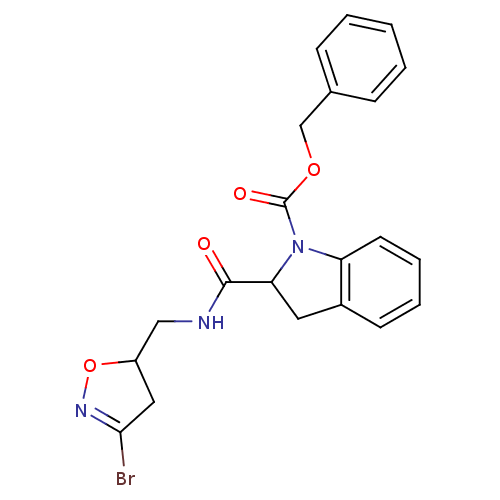
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
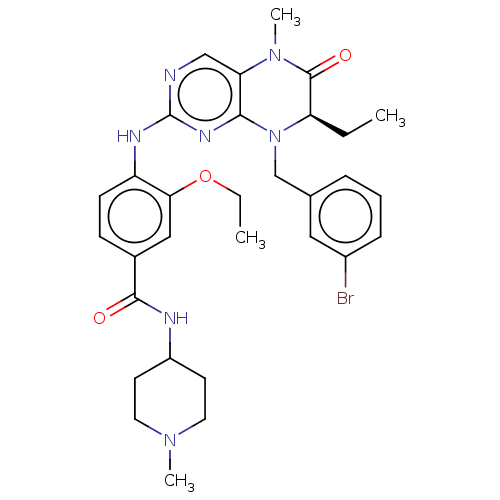
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
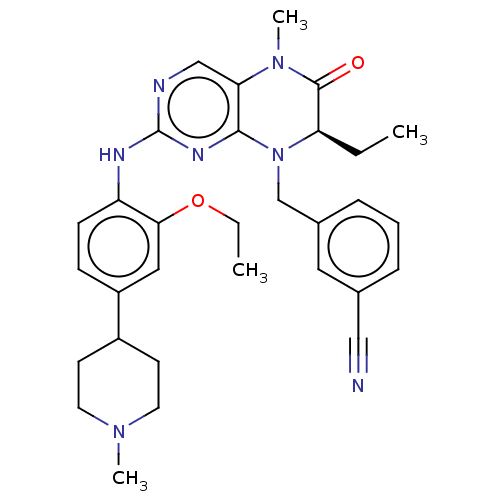
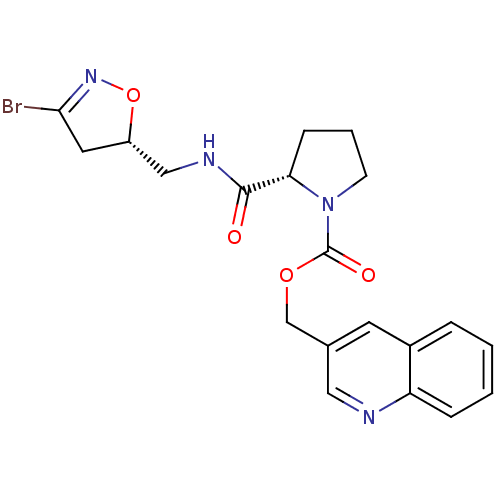
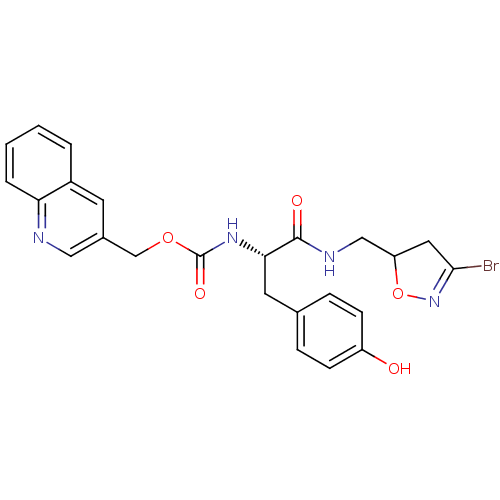
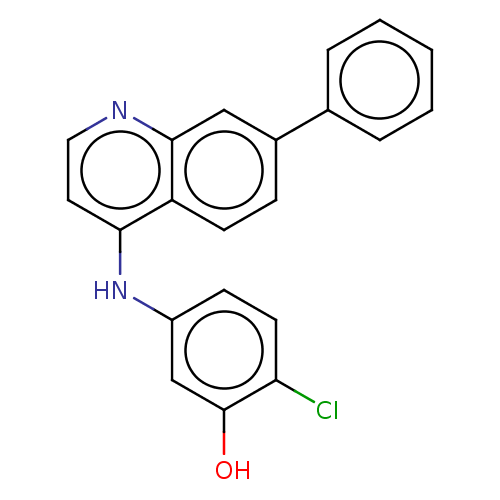
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
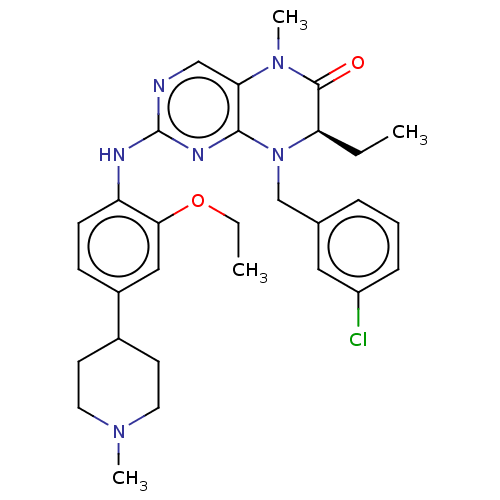
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
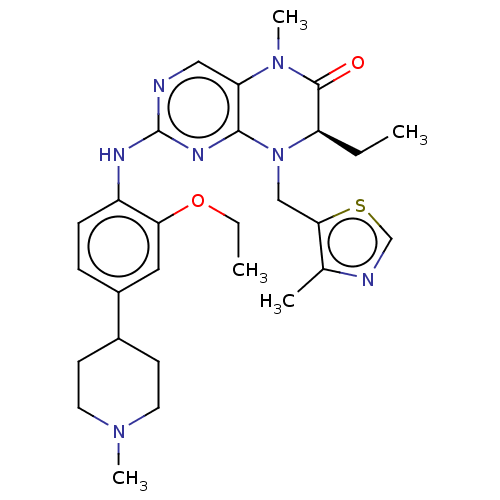
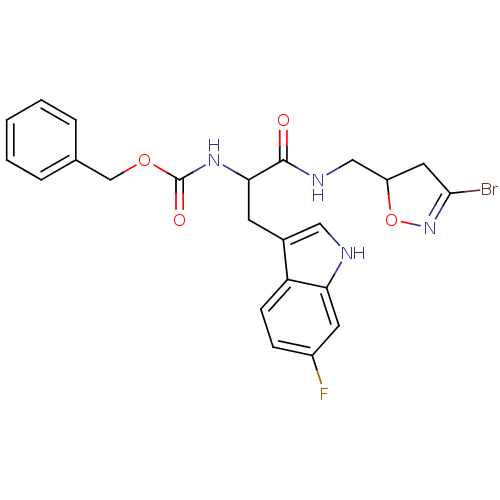
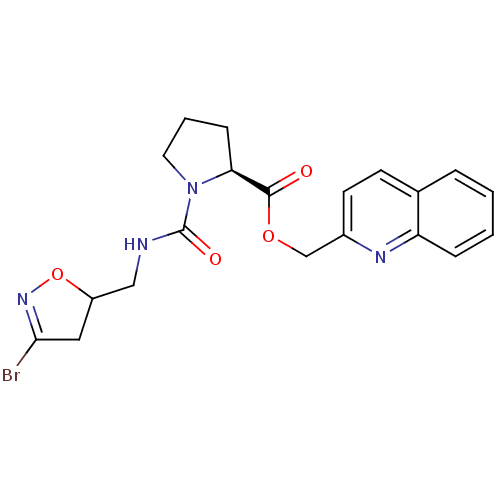
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.90E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 3.40E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 4.10E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 5.10E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 6.20E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 6.80E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 9.90E+4nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetProtein-glutamine gamma-glutamyltransferase 2(Homo sapiens (Human))
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataKi: 1.38E+5nMAssay Description:Inhibition of human recombinant TG2 expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of VEGFR2 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of RIP2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of RIP2 (unknown origin) by biochemical assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 9.90nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of VEGFR2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of RIP2 (unknown origin)More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 68nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 79nMAssay Description:Inhibition of ALK F1174L mutant auto-phosphorylation in human Kelly cells after 3 hrs by MSD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of ALK5 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 84nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 85nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 125nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assayMore data for this Ligand-Target Pair
TargetBromodomain-containing protein 4(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme...More data for this Ligand-Target Pair
TargetBromodomain-containing protein 4(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibition of ALK (unknown origin)More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 440nMAssay Description:Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
The Institute of Cancer Research
Curated by ChEMBL
The Institute of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of full-length C-terminal NanoLuc-tagged ALK (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engagement as...More data for this Ligand-Target Pair







 3D Structure (crystal)
3D Structure (crystal)