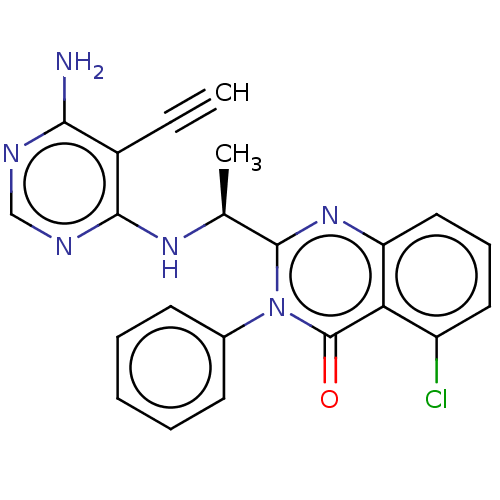
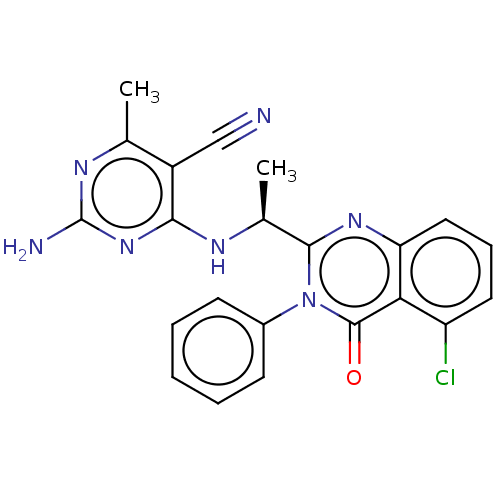
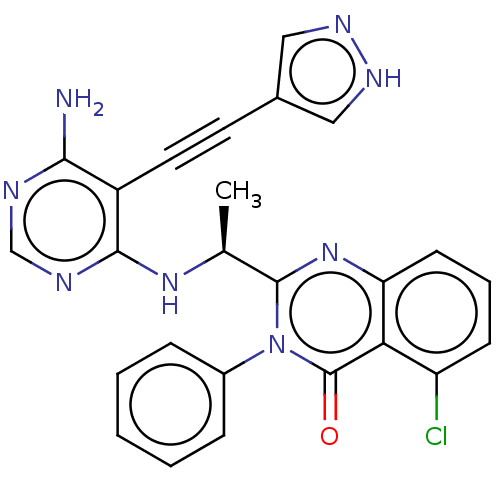
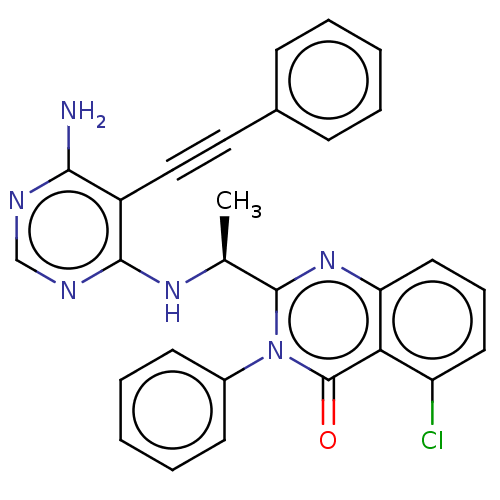
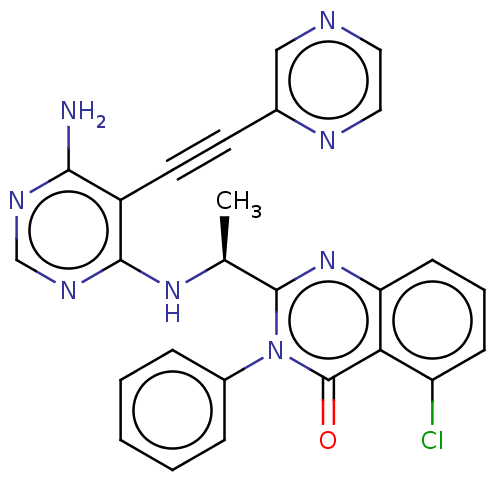
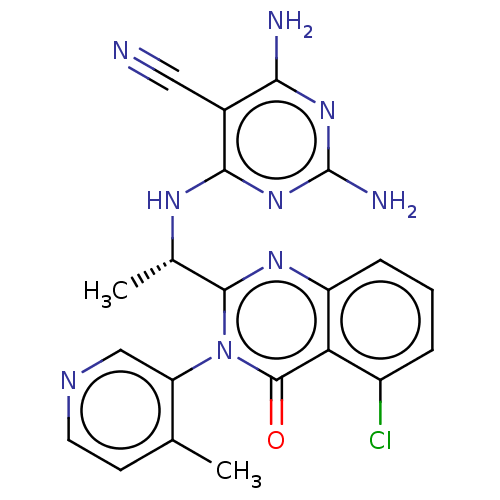
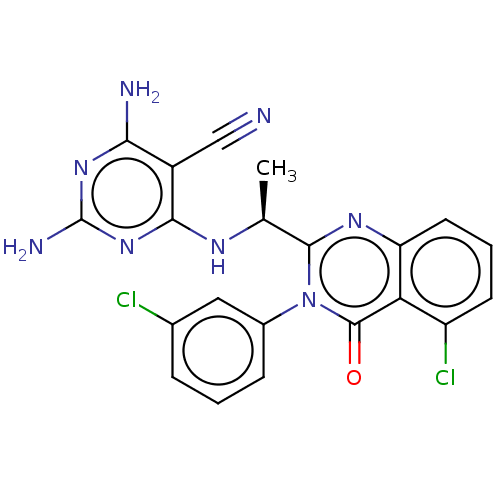
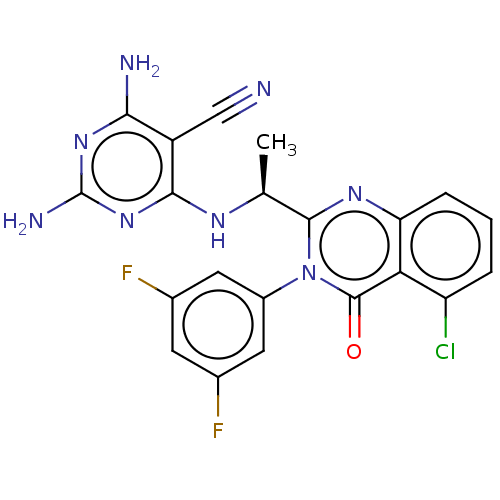
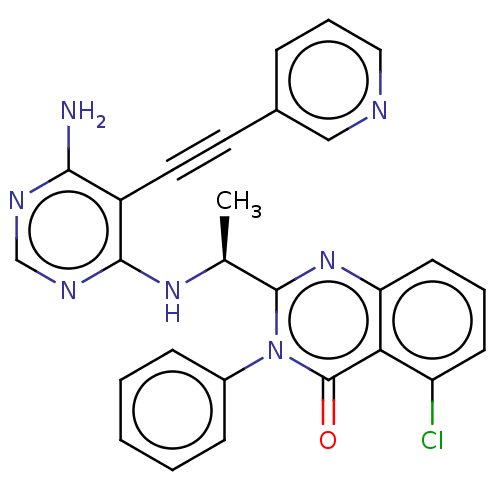
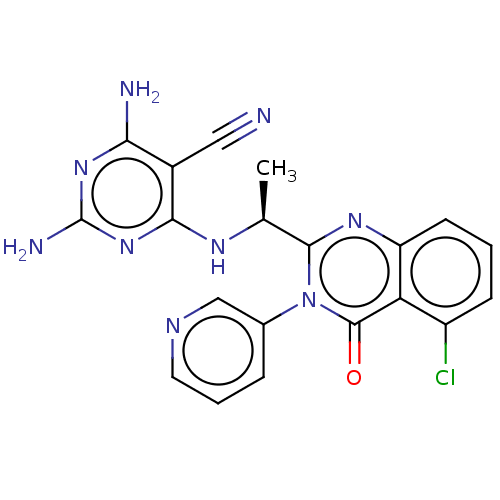
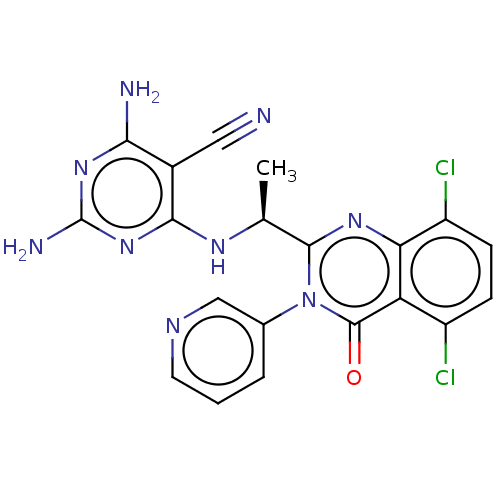
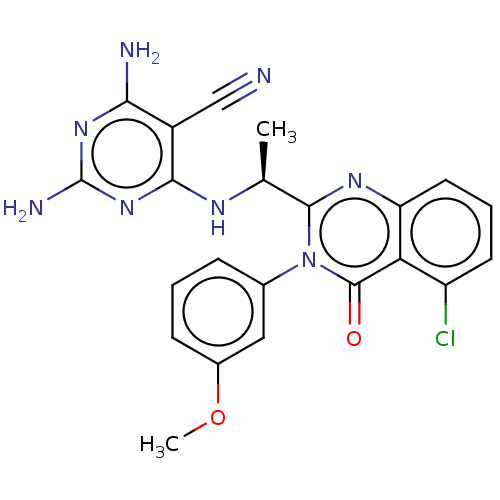
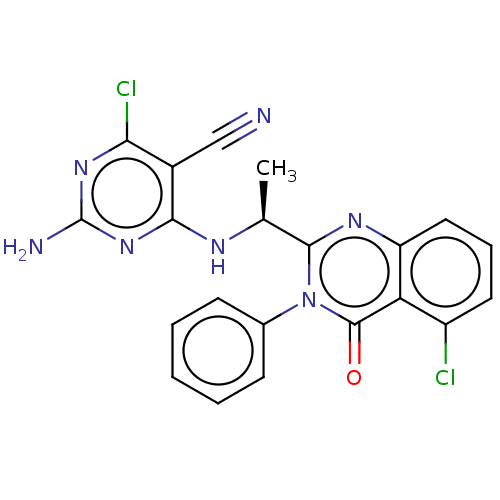
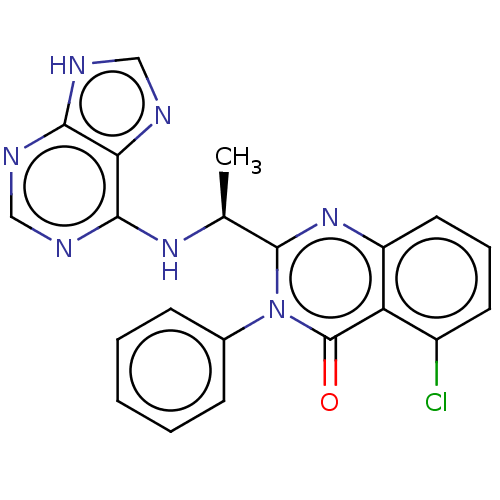
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
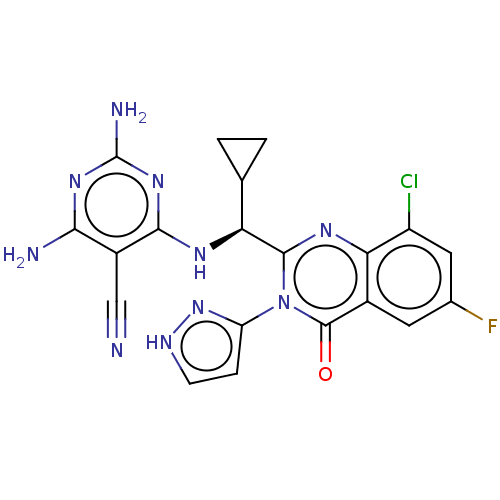
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci...More data for this Ligand-Target Pair
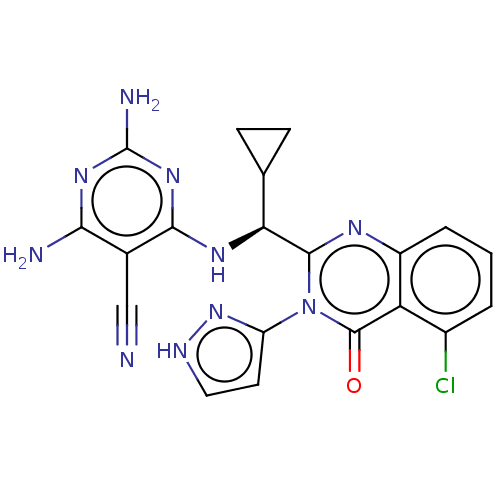
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci...More data for this Ligand-Target Pair
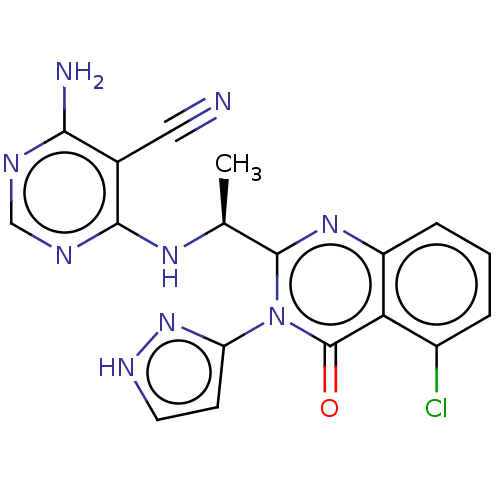
Affinity DataKi: 5.20E+3nMAssay Description:Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+4nMAssay Description:Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 1(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 5.10E+4nMAssay Description:Binding affinity to recombinant human ENT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 2(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 1.06E+5nMAssay Description:Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+5nMAssay Description:Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 2(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 1.89E+5nMAssay Description:Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKi: 2.31E+5nMAssay Description:Binding affinity to recombinant human CNT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetEquilibrative nucleoside transporter 2(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataKi: 2.42E+5nMAssay Description:Binding affinity to recombinant human ENT2 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.150nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.450nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.470nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.540nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of N-terminal His6-tagged recombinant full-length human PI3K p110beta/untagged recombinant full length human p85alpha expressed in baculov...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human full length PI3K p110delta/p85 alpha using PIP2/ATP as substrate after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human full length PI3K p110delta/p85alpha using phosphatidylinositol 3,4,5-trisphosphate as substrate measured after 30 mins by TR-FRET...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Gilead Sciences, Inc.
Curated by ChEMBL
Gilead Sciences, Inc.
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition human full length PI3Kdelta catalytic subunit/p85alpha assessed as formation of PIP3 after 30 mins by europium labeled GRP-based TR-FRET a...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)