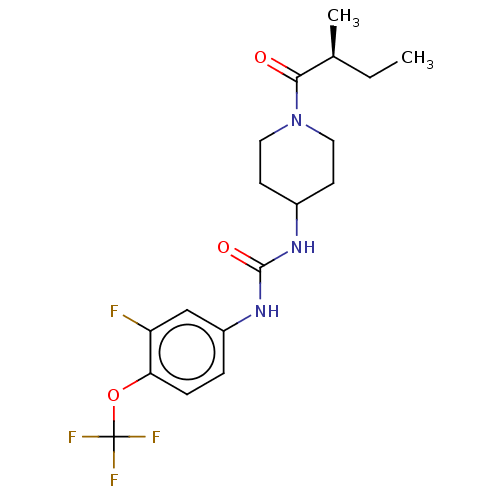
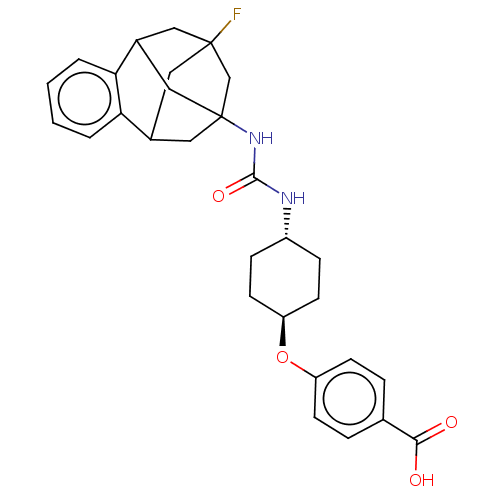
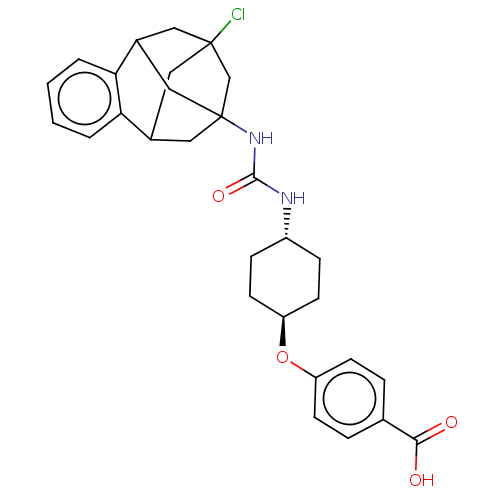
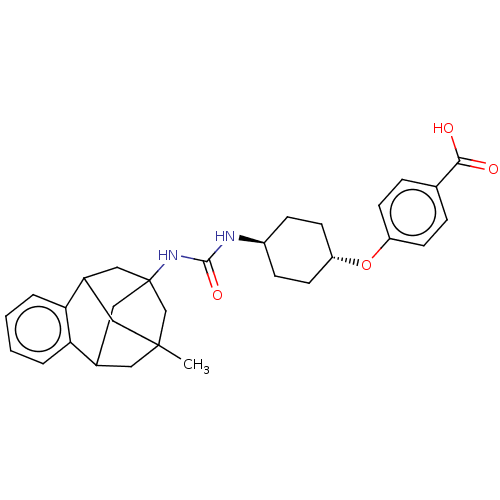
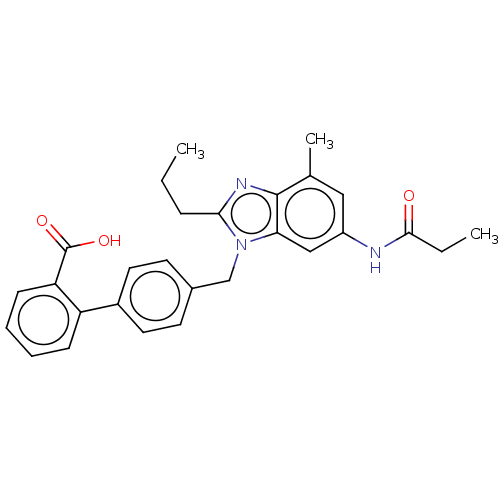
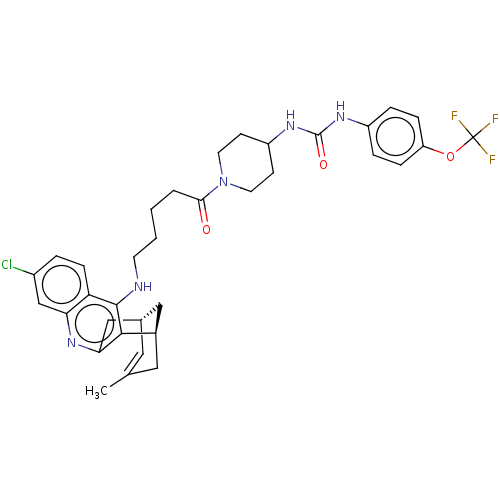
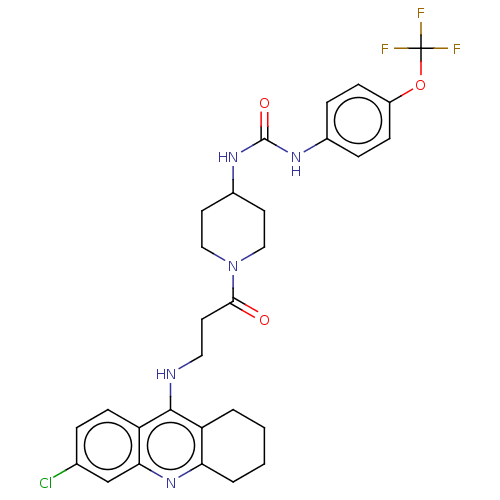
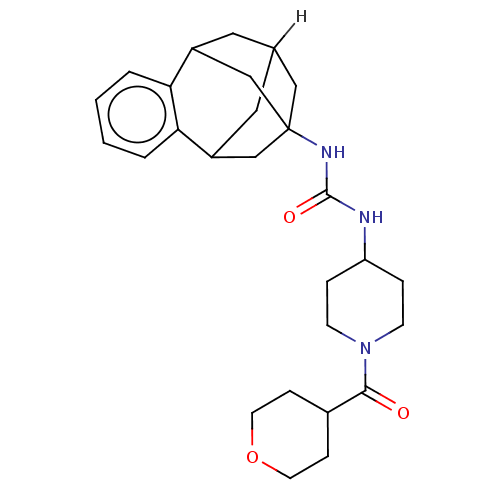
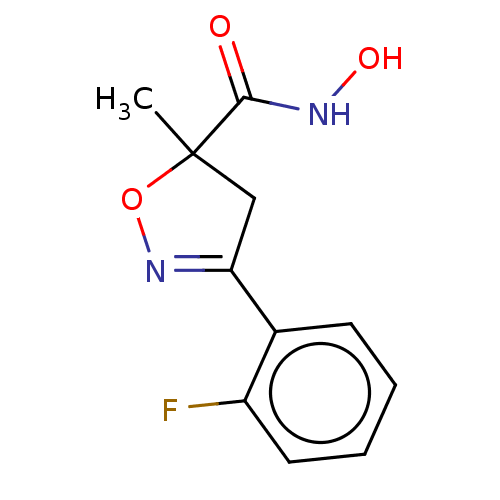
Affinity DataKi: 133nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 257nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 297nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 516nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 598nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 612nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 641nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 733nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 802nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 808nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 815nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 847nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.24E+3nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.81E+3nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.79E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.73E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.76E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.40E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.22E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.45E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant carbonic anhydrase-1-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant carbonic anhydrase-2-mediated CO2 hydration by stopped-flow assayMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
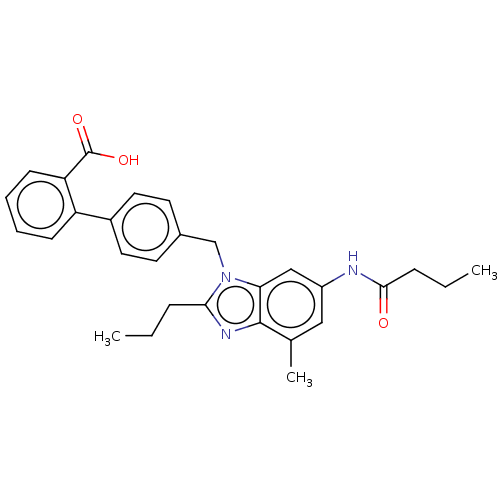
Affinity DataIC50: 0.100nMAssay Description:Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysisMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysisMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Inhibition of angiotensin AT1 receptorMore data for this Ligand-Target Pair
TargetType-2 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of rat recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of mouse recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
TargetType-1 angiotensin II receptor(Homo sapiens (Human))
Beijing Institute of Technology
Curated by ChEMBL
Beijing Institute of Technology
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysisMore data for this Ligand-Target Pair

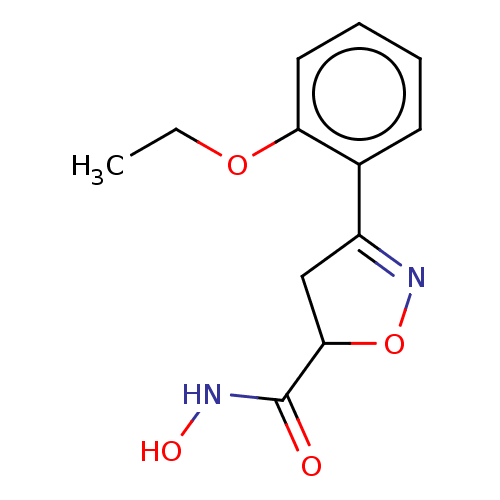
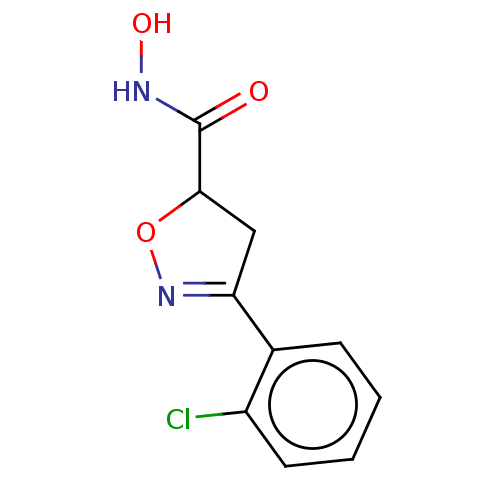
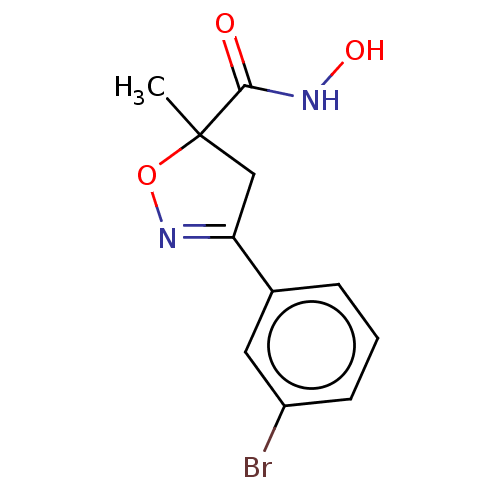
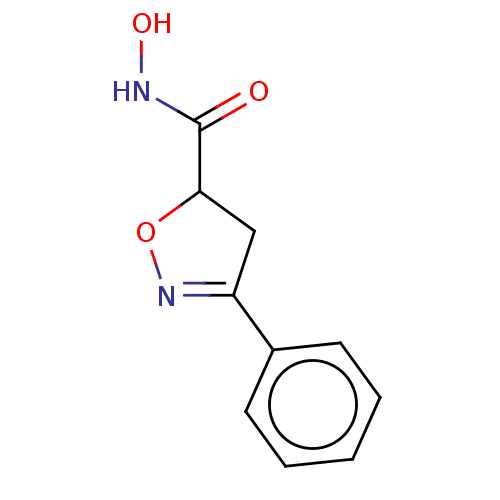




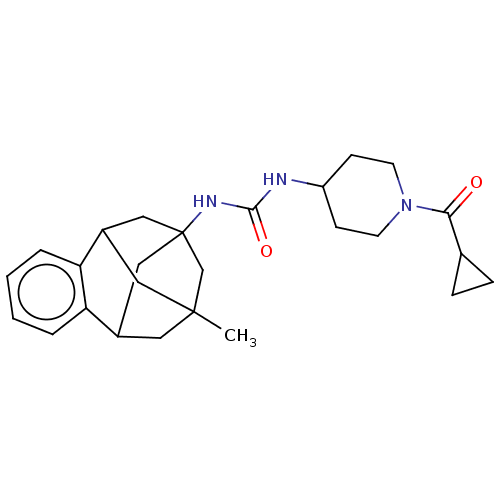
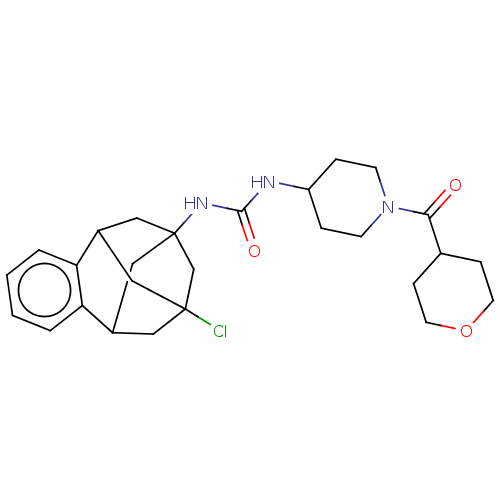
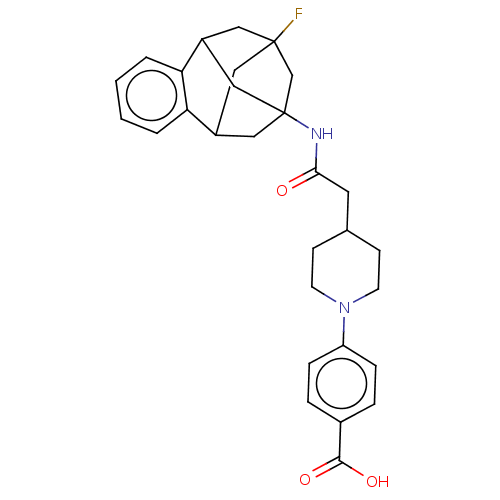

 3D Structure (crystal)
3D Structure (crystal)