TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Mixed-type inhibition of human AChE assessed as hydrolysis of acetylthiocholine by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human recombinant AChE by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.290nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.370nMAssay Description:Inhibition of human recombinant AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.430nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.570nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human serum BChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.670nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.690nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.740nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.810nMAssay Description:Mixed-type inhibition of human AChE assessed as hydrolysis of acetylthiocholine by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.820nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 0.820nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.880nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 0.890nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.06nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Mixed-type inhibition of human AChE assessed as hydrolysis of acetylthiocholine by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.41nMAssay Description:Inhibition of human recombinant AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate after 20 mins preincubation by Ellman methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 1.74nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 1.86nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 2.16nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 2.28nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 3.32nMAssay Description:Inhibition of human recombinant AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 4.04nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 4.83nMAssay Description:Inhibition of human recombinant AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 5.13nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human serum recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Inhibition of rat cortex AChEMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 5.73nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 5.73nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Rattus norvegicus (rat))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of rat cortex AChEMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 7.03nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 7.25nMAssay Description:Inhibition of human serum BChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human recombinant AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetCholinesterase(Homo sapiens (Human))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 8.06nMAssay Description:Inhibition of human serum BChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Bos taurus (bovine))
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC)
Curated by ChEMBL
Affinity DataIC50: 8.12nMAssay Description:Inhibition of bovine erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 8.32nMpH: 8.0 T: 2°CAssay Description:AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Institut f£r Molekulare Physiologie
Curated by ChEMBL
Affinity DataIC50: 8.32nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's assayMore data for this Ligand-Target Pair


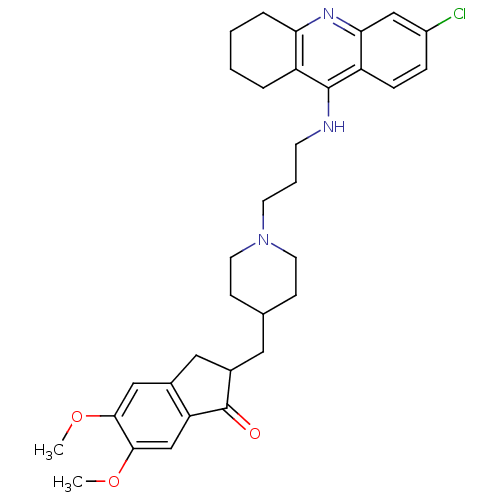

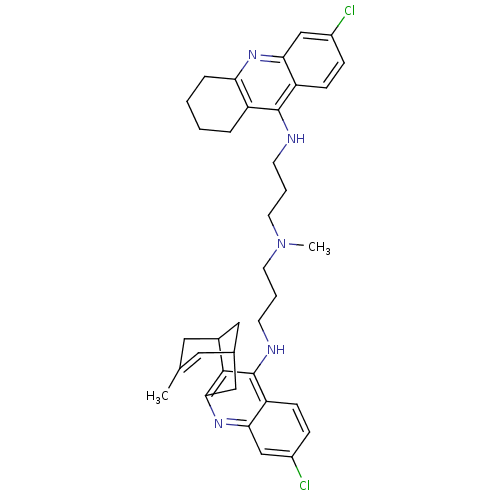

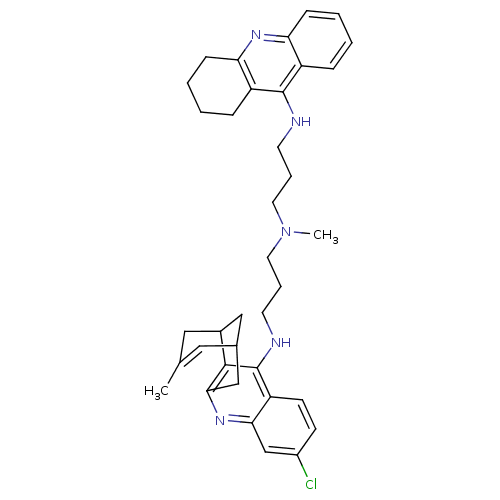

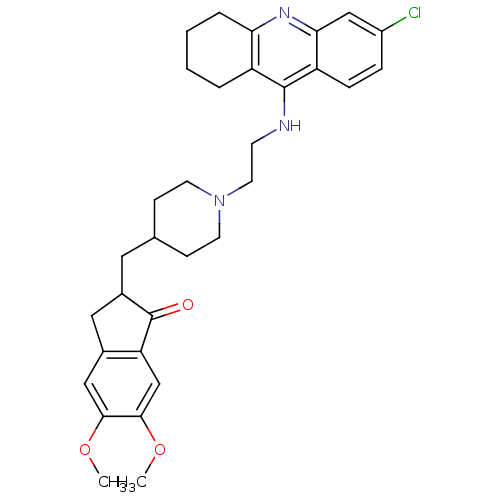







 3D Structure (crystal)
3D Structure (crystal)