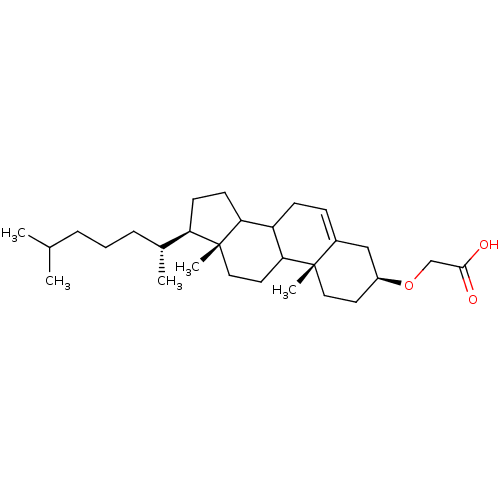
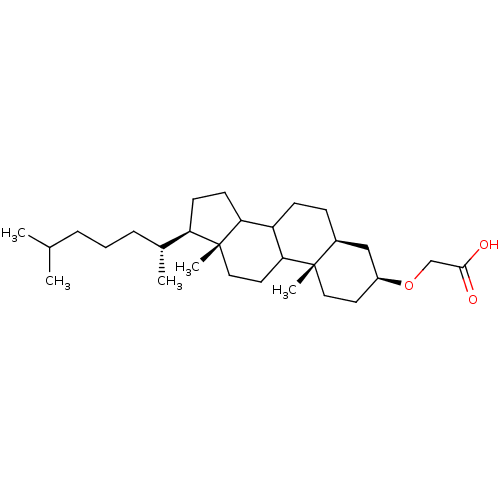
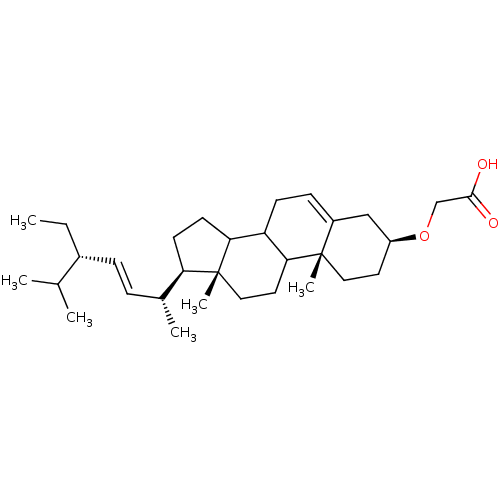
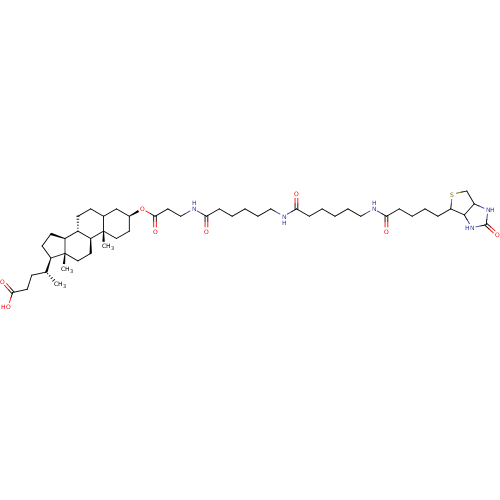
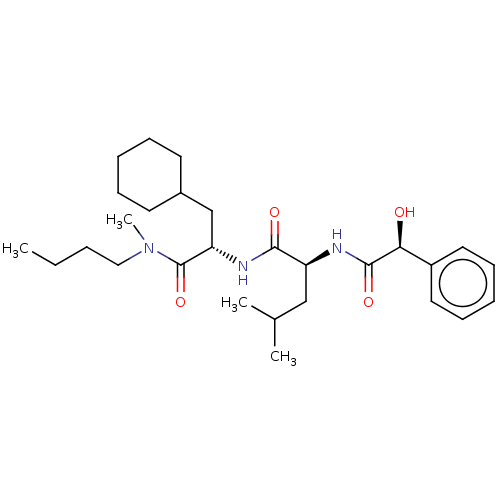
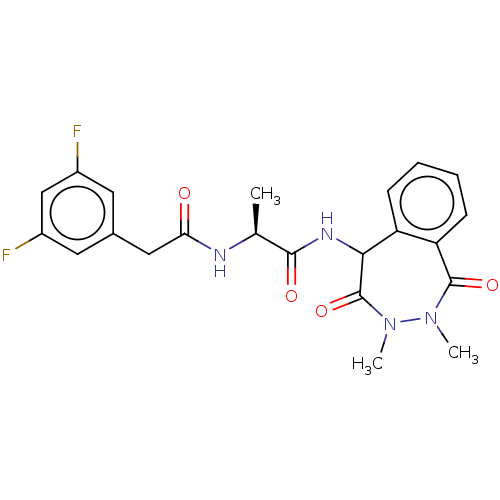
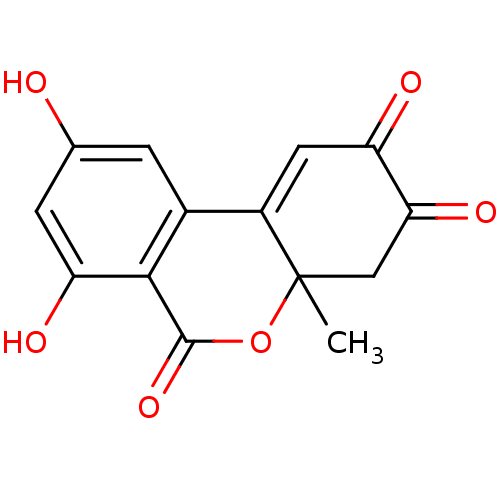
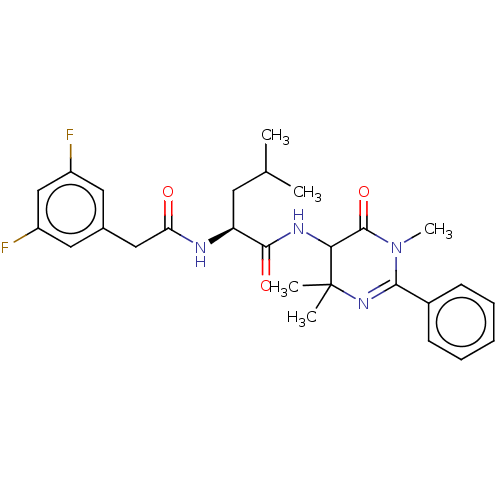
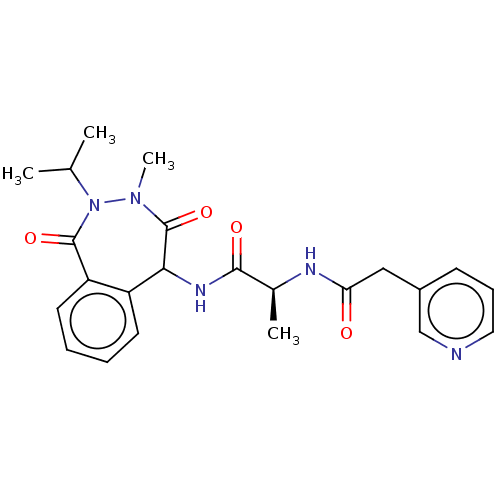
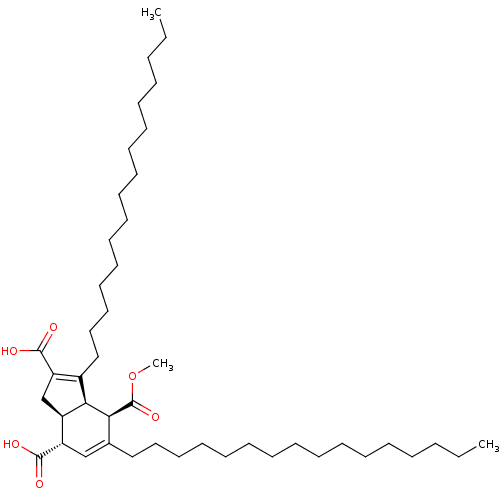
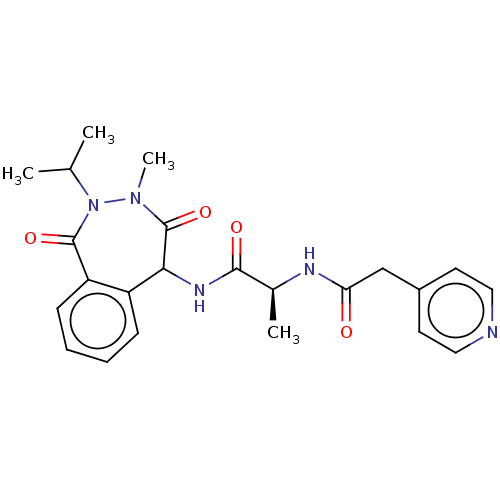
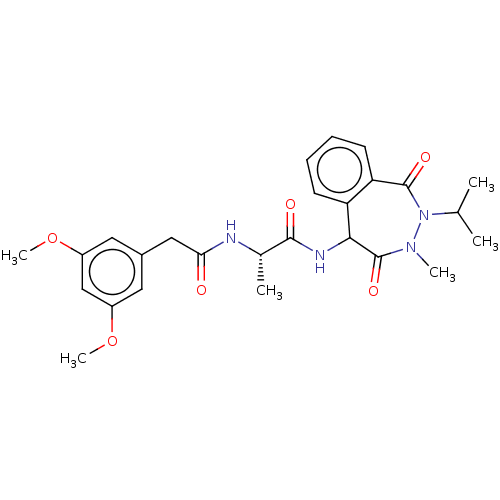
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 3.75E+3nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+3nMAssay Description:Competitive inhibition of recombinant rat DNA polymerase beta using poly(dA)/oligo(dT)18 as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of nucleotide substrate c...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 7.84E+3nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
Affinity DataKi: 9.85E+3nMAssay Description:Competitive inhibition of human C-terminal-His6-tagged DNA polymerase kappa expressed in Escherichia coli using dTTP as substrate by Dixon plot analy...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.12E+4nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.16E+4nMAssay Description:Noncompetitive inhibition of recombinant rat DNA polymerase beta using dTTP as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.28E+4nMAssay Description:Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.38E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
Affinity DataKi: 1.81E+4nMAssay Description:Non-competitive inhibition of human C-terminal-His6-tagged DNA polymerase kappa expressed in Escherichia coli using ploy(dA)/oligo(dT)18 as substrate...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.85E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
Affinity DataKi: 2.01E+4nMAssay Description:Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 2.79E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
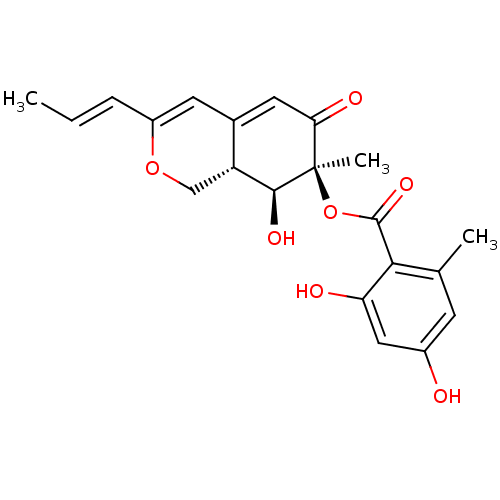
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 71nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 76nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 180nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
Affinity DataIC50: 680nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 720nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 890nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetDNA nucleotidylexotransferase(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity of the compound against human terminal deoxynucleotidyltransferaseMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetGamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2(Homo sapiens (Human))
Tokyo University of Science
Curated by ChEMBL
Tokyo University of Science
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human gamma-secretase activityMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory concentration against human DNA polymerase alpha incubated with 0.05 unitsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibitory effect of the compound on the activity of rat DNA polymerase beta was determined by inhibitory dose curvesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMpH: 7.5 T: 2°CAssay Description:For the DNA polymerase reaction, the dehydroaltenusin derivative was dissolved in dimethyl sulfoxide (DMSO) at various concentrations and sonicated f...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibitory concentration against rat DNA polymerase beta incubated with 0.05 unitsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.19E+4nMAssay Description:Inhibition of rat DNA polymerase betaMore data for this Ligand-Target Pair