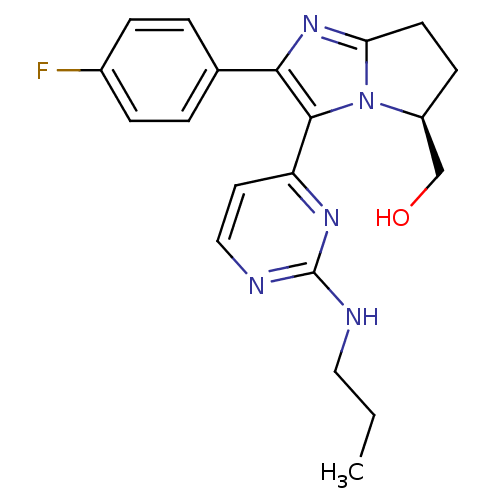
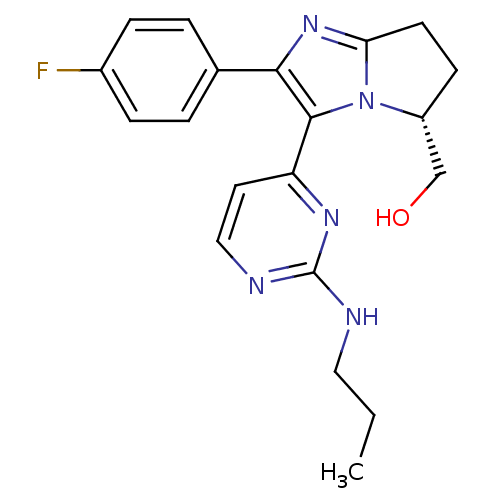
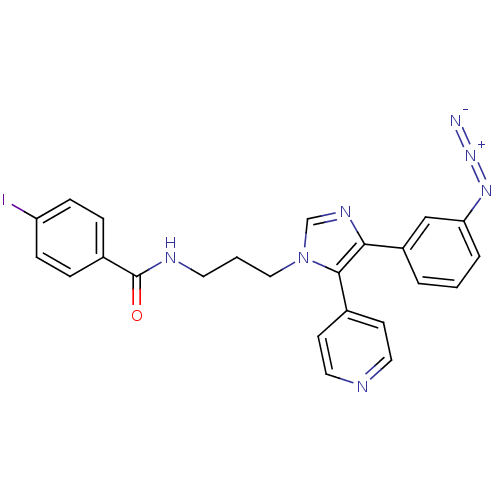
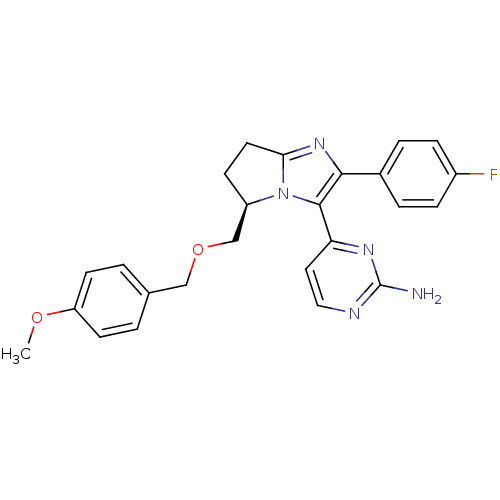
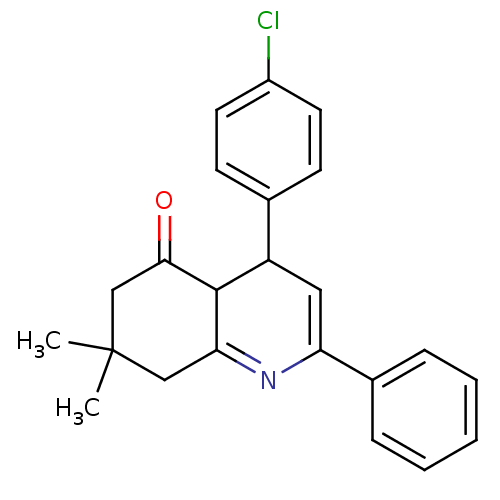
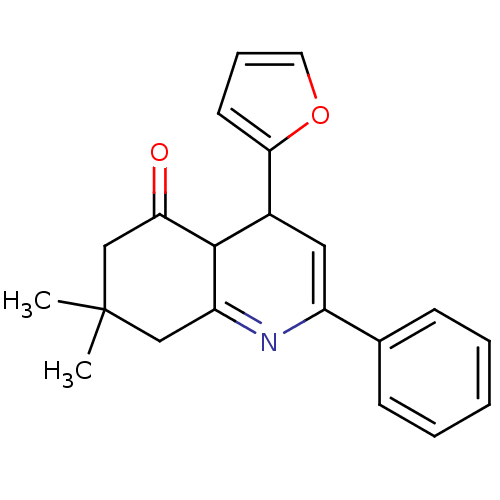
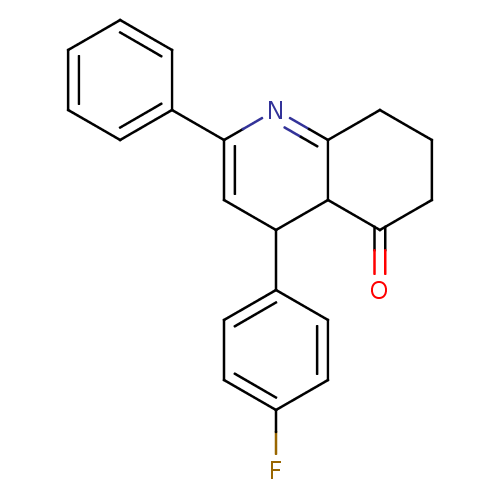
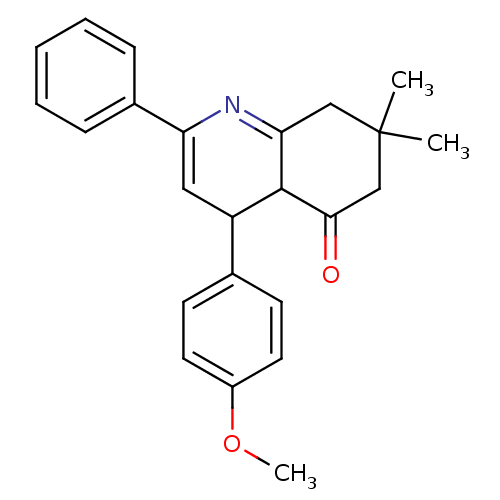
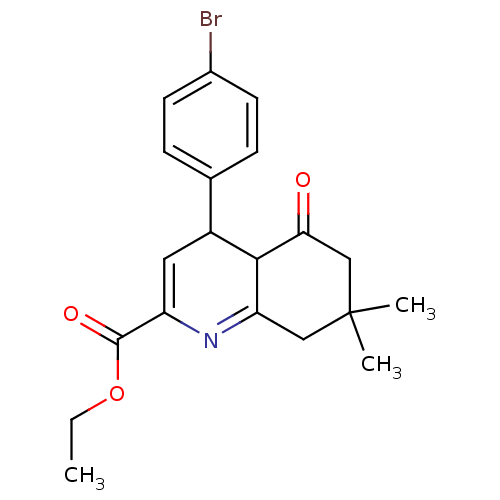
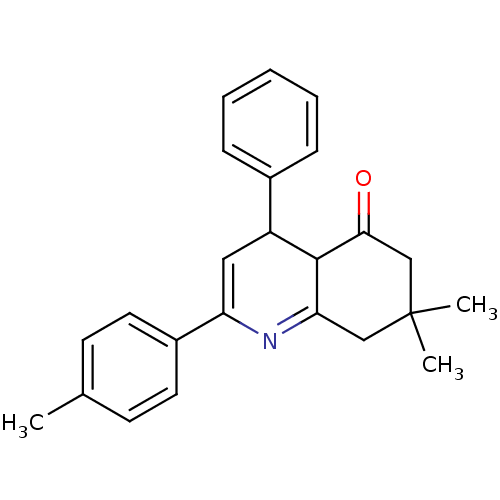
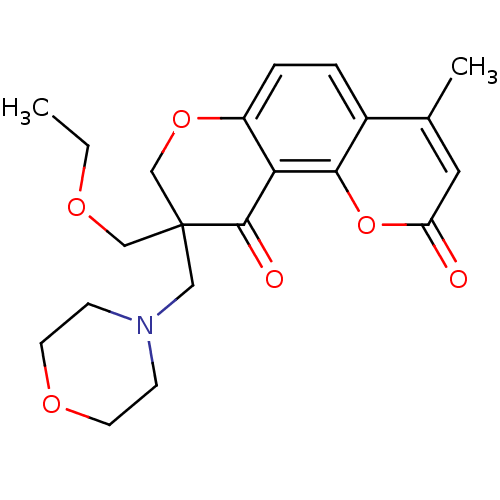
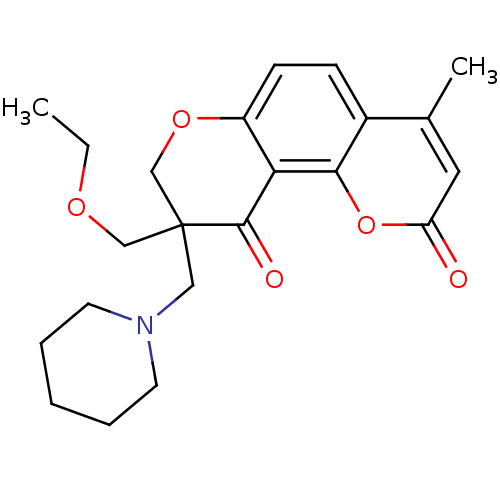
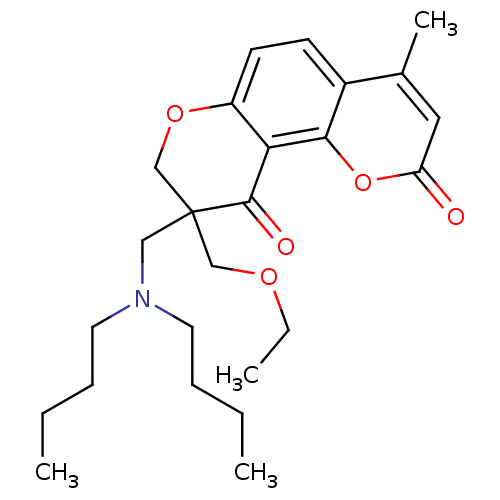
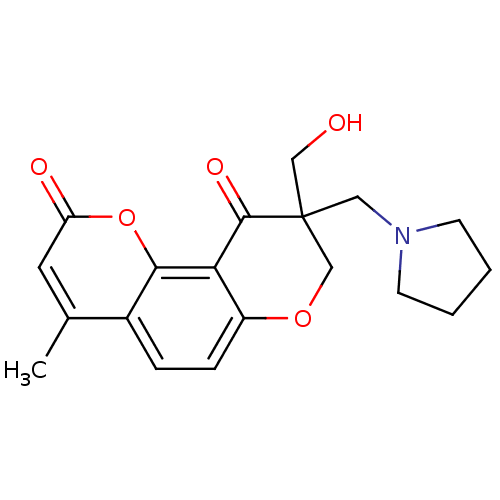
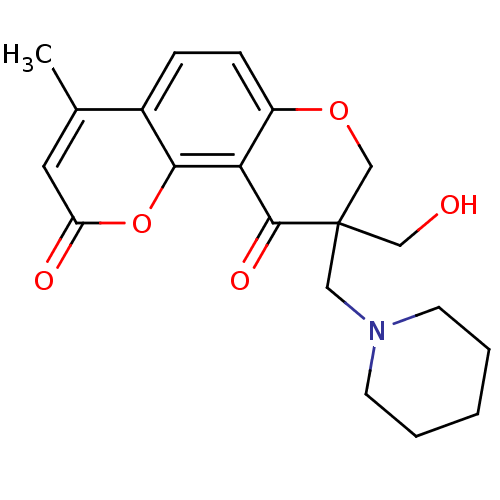
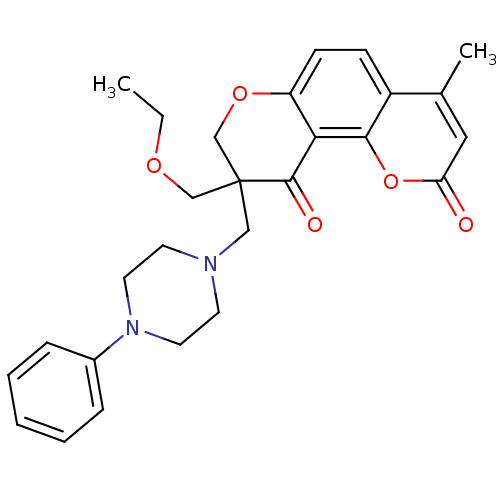
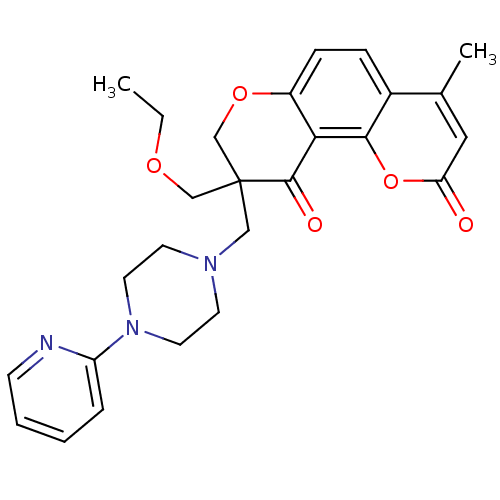
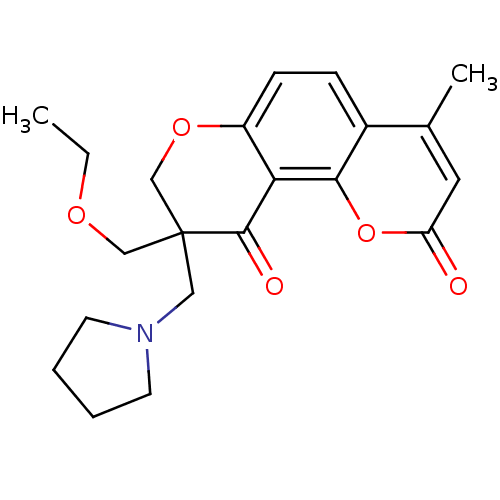
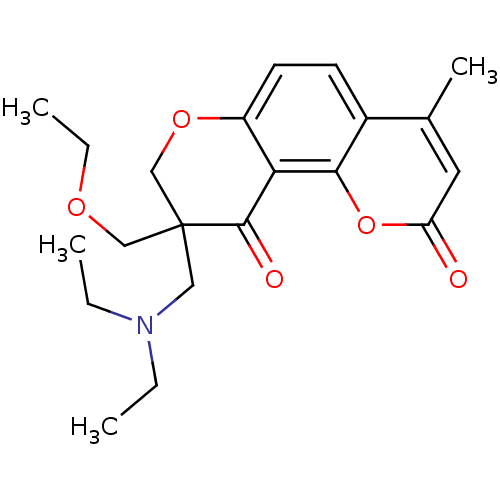
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
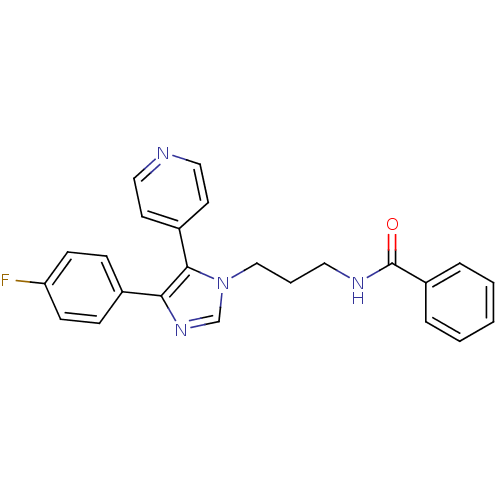
Affinity DataIC50: 2.5nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
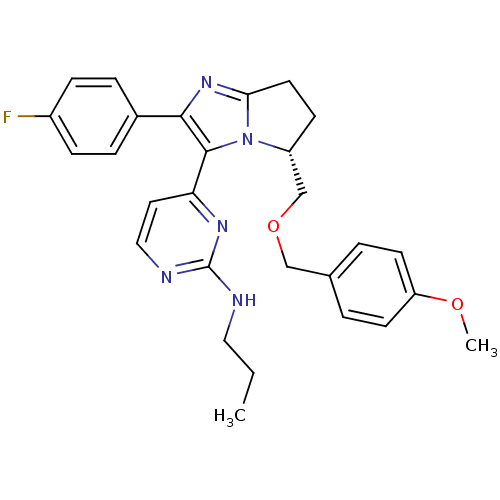
Affinity DataIC50: 3nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
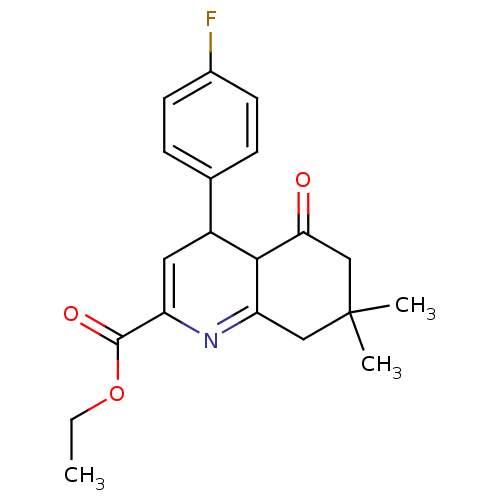
Affinity DataIC50: 10.9nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
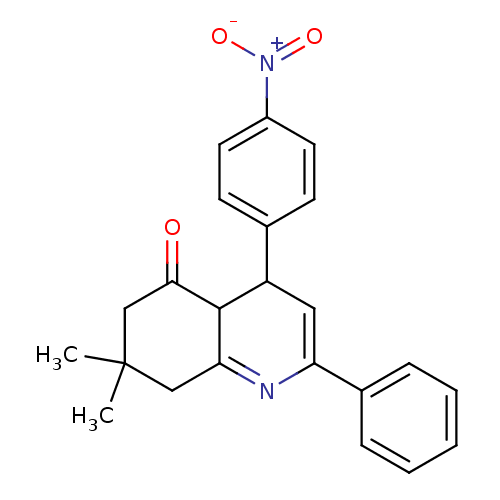
Affinity DataIC50: 28nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibitory concentration against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 61.2nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 116nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibitory concentration against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 217nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 233nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 295nMAssay Description:Inhibitory activity against p38 at a concentration of 1.0 uM More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.70E+3nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.45E+4nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.66E+4nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.96E+4nMAssay Description:Inhibition of PTP1B assessed as p-nitrophenolate ion production pretreated for 10 mins measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.45E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.62E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.05E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.24E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.67E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.21E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.55E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.06E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.62E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.27E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Central Drug Research Institute
Curated by ChEMBL
Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.04E+4nMAssay Description:Inhibition of PTP1BMore data for this Ligand-Target Pair