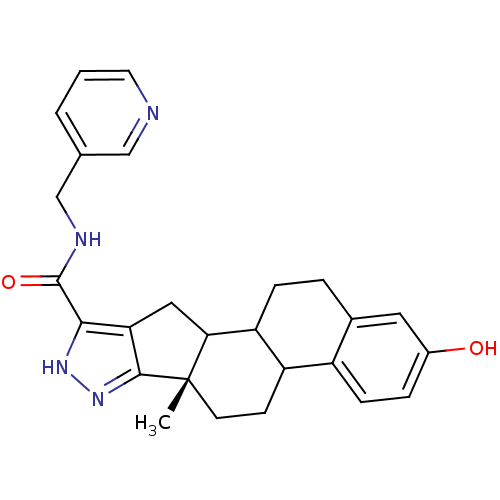
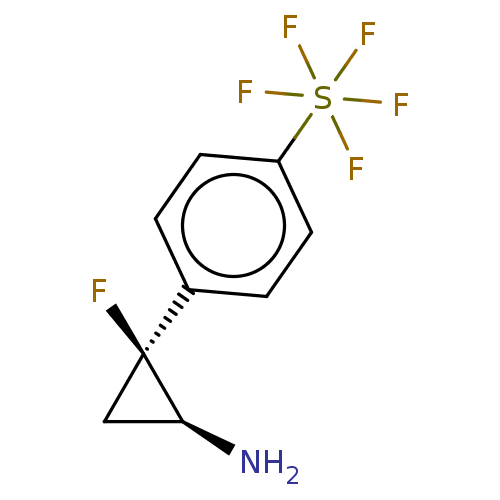
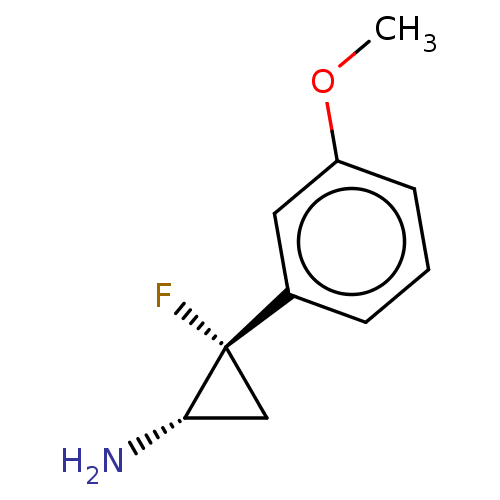
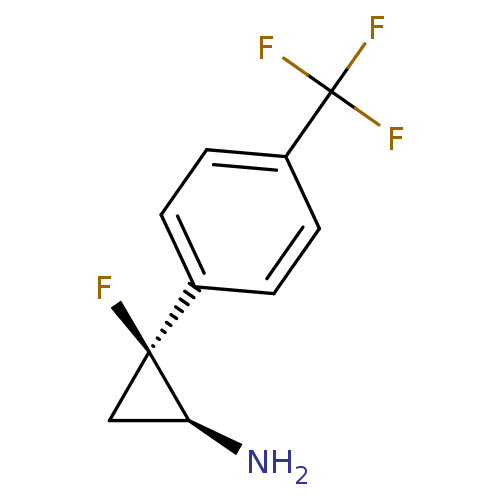
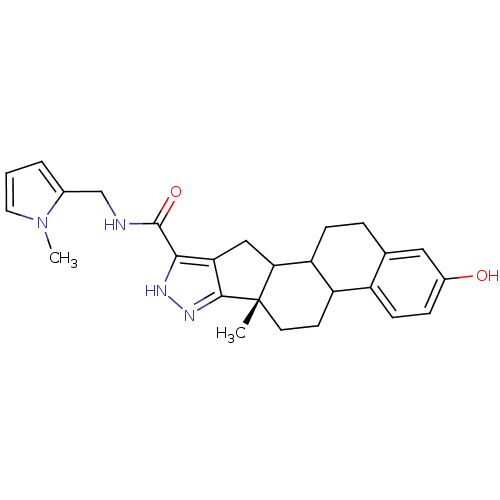
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 6.80E+3nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 8.90E+3nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 1.17E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.10E+4nMAssay Description:Inhibition of human LSD1More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.50E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.66E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 2.81E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 4.18E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 7.96E+4nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant His-tagged full length LSD1 expressed in Escherichia coli BL21 (DE3) assessed as inactivation constant preincubated f...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 1.68E+5nMAssay Description:Inhibition of human truncated LSD1 lacking N-terminal 184 amino acidsMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataKi: 1.68E+5nMAssay Description:Inhibition of human truncated LSD1 lacking N-terminal 184 amino acidsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Affinity DataIC50: 3.97nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMT: 2°CAssay Description:In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 17.5nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Affinity DataIC50: 21.6nMT: 2°CAssay Description:In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 47nMT: 2°CAssay Description:In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Affinity DataIC50: 126nMT: 2°CAssay Description:In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Affinity DataIC50: 196nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 530nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Affinity DataIC50: 775nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 780nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Affinity DataIC50: 787nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of recombinant human His-tagged LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate preincubated for 10 mins ...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Affinity DataIC50: 881nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Affinity DataIC50: 897nMpH: 8.0 T: 2°CAssay Description:For the enzyme assay, the enzyme fraction was added to fluorescent substrate in the presence of test compounds, and the mixture was incubated for add...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 920nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of recombinant human His-tagged LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate preincubated for 10 mins ...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
University of Southampton
Curated by ChEMBL
University of Southampton
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of recombinant human His-tagged LSD1 expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate preincubated for 10 mins ...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University of Bath
Curated by ChEMBL
University of Bath
Curated by ChEMBL
Affinity DataIC50: 2.75E+3nMAssay Description:Inhibition of 17-beta-hydroxysteroid dehydrogenase type 1 expressed in T47D human breast cancer cells using 2 nM [3H]estroneMore data for this Ligand-Target Pair

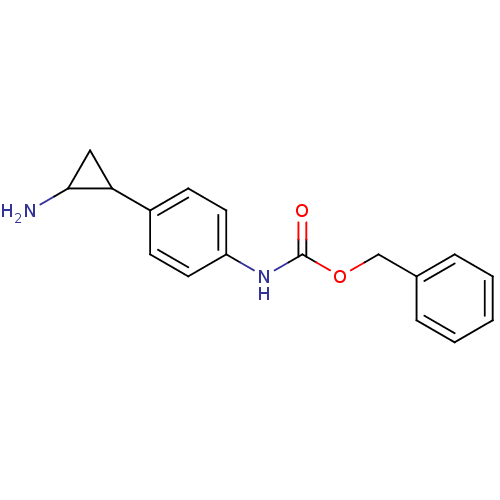
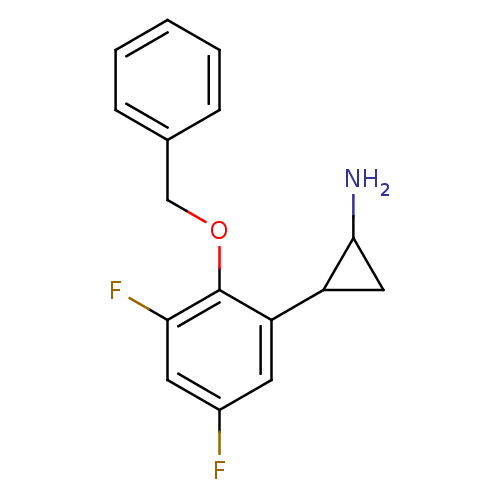
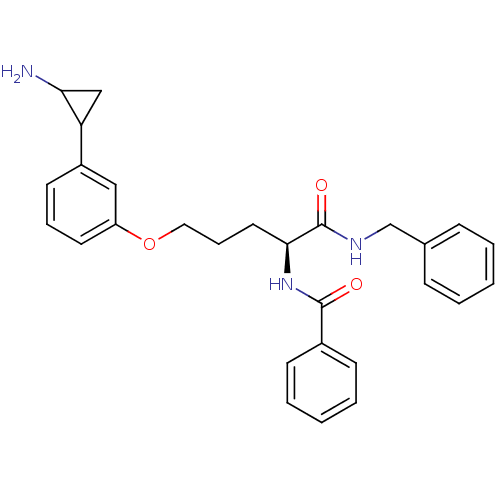
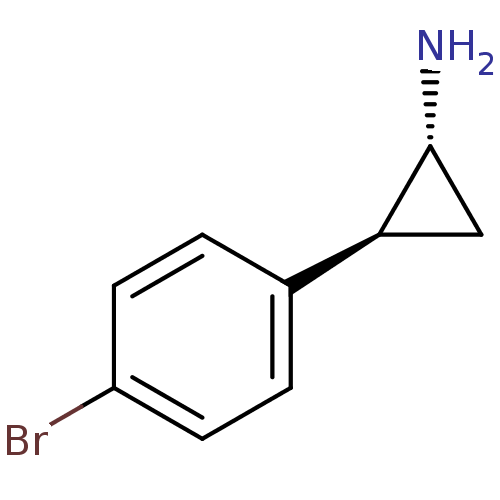
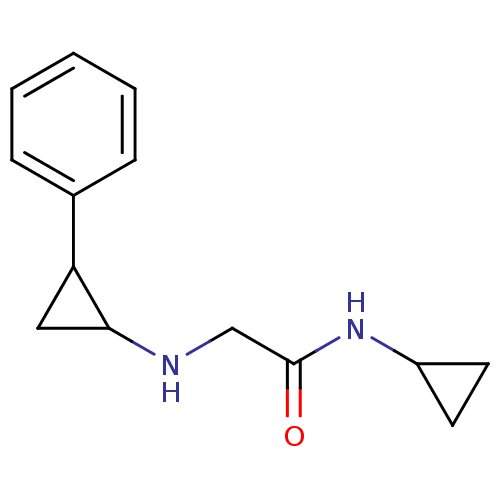

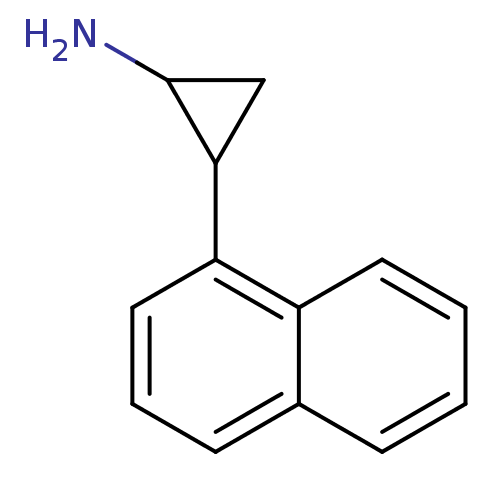
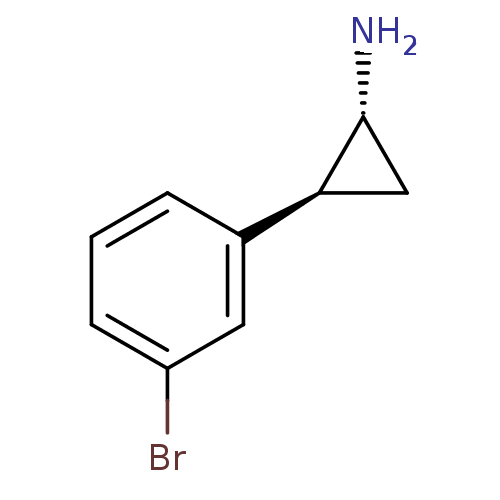
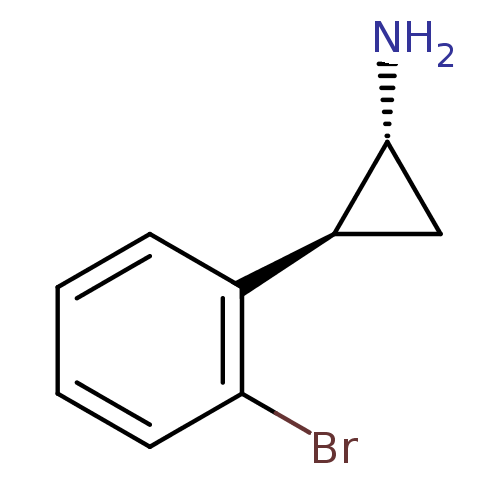


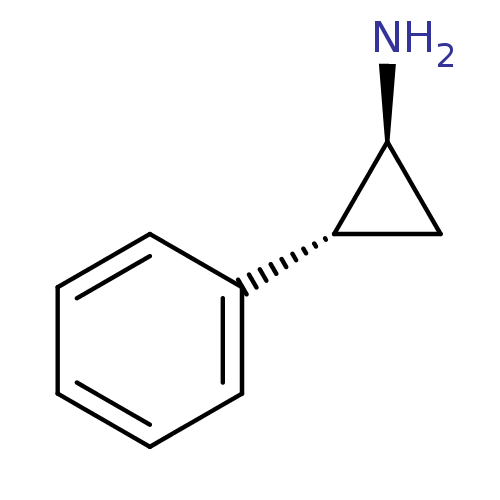
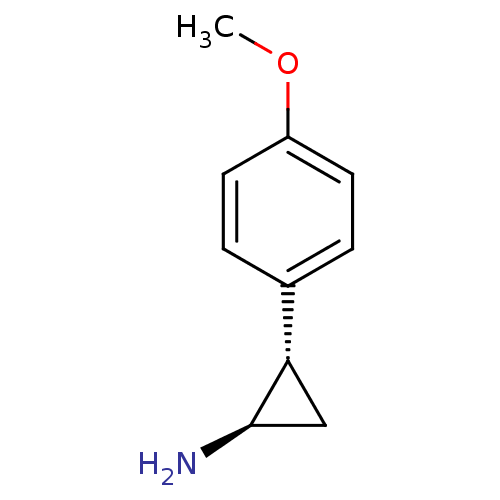





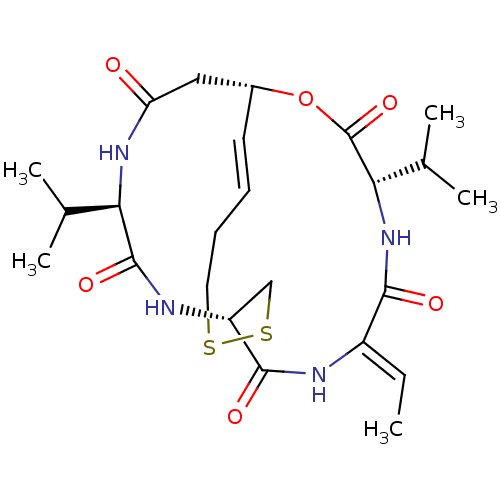
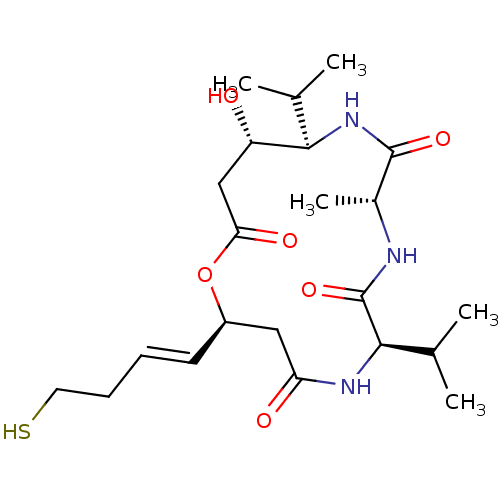

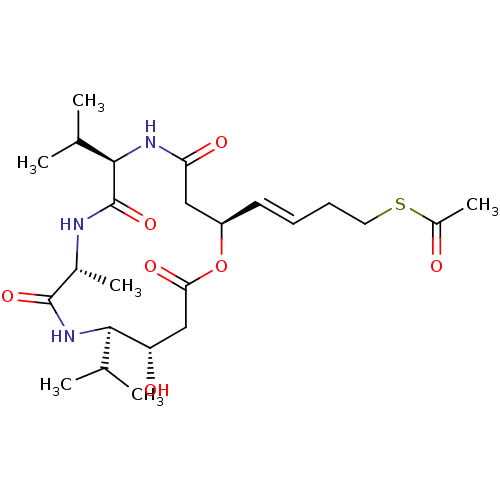
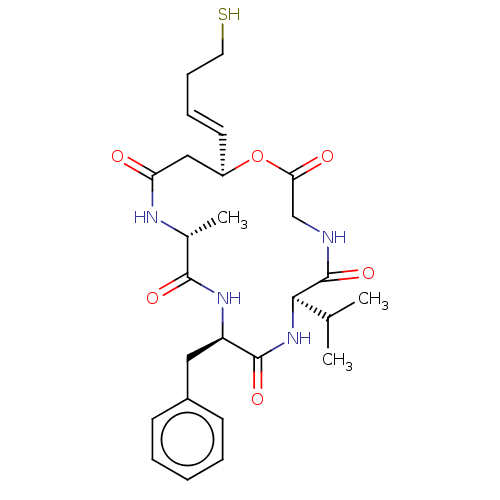
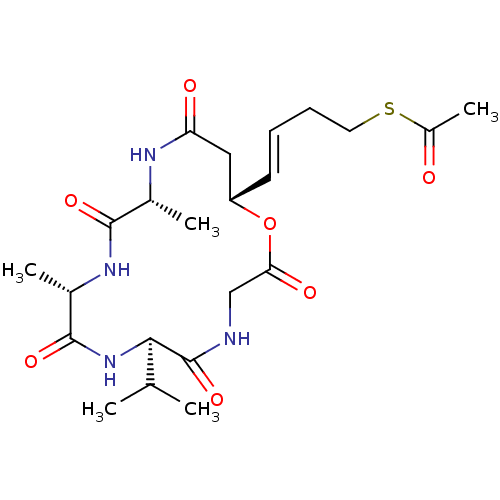


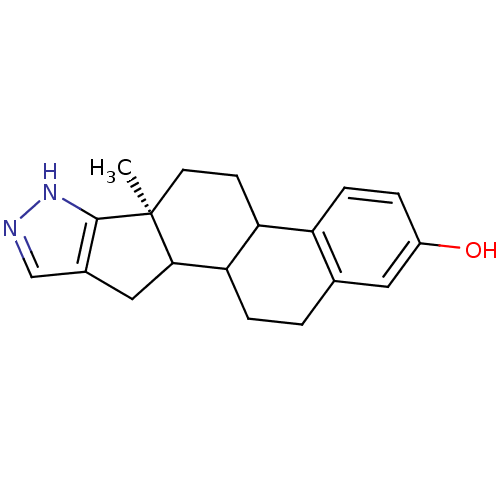


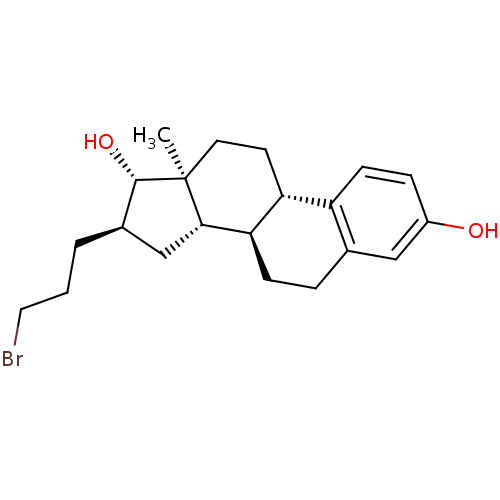
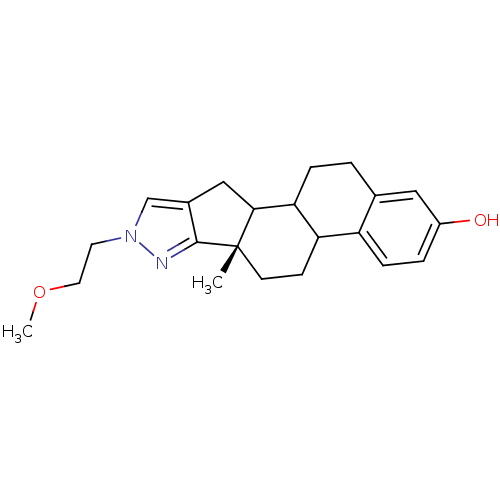

 3D Structure (crystal)
3D Structure (crystal)