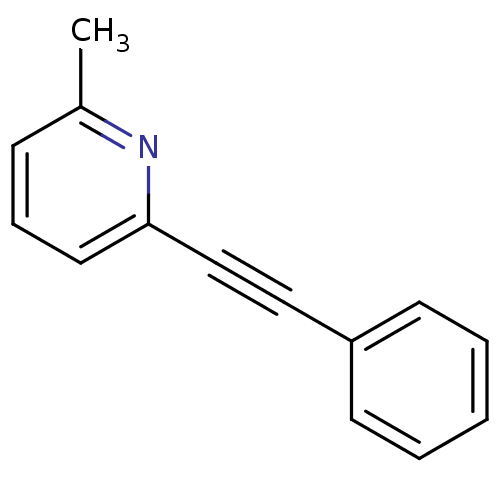
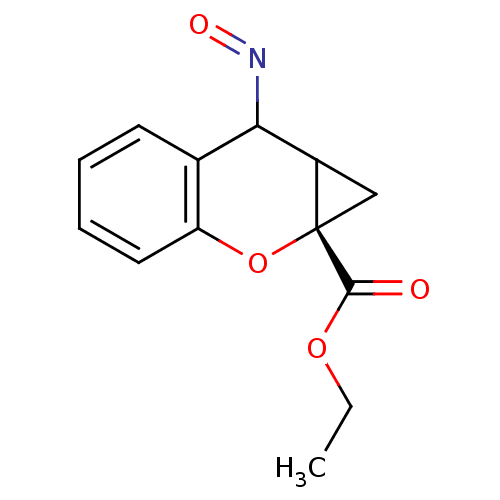
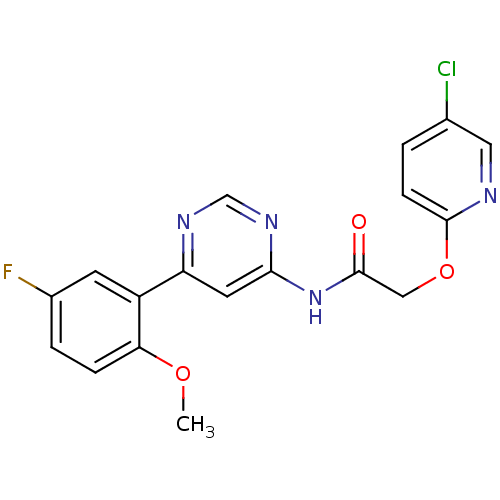
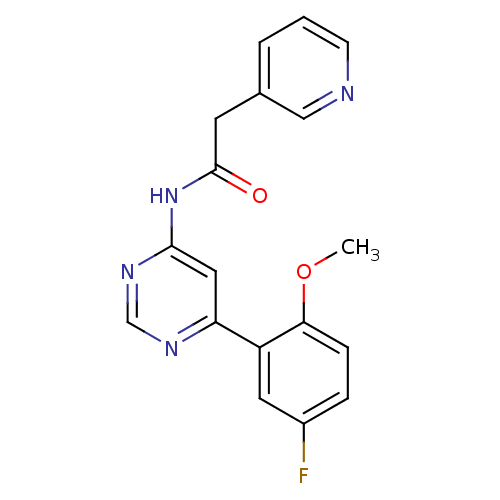
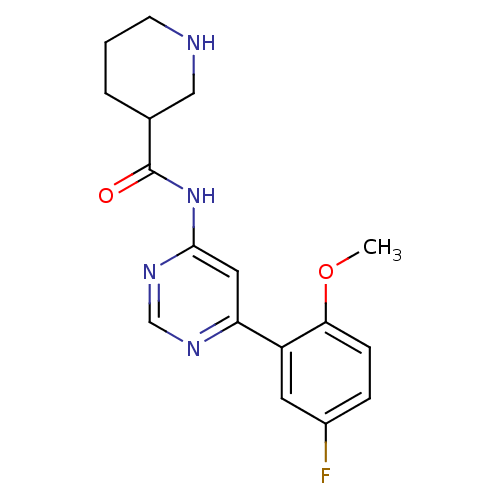
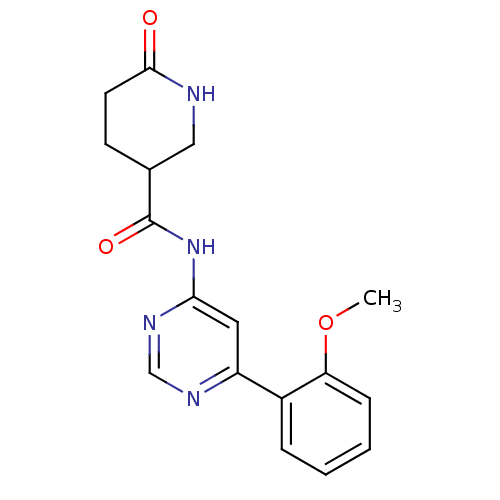
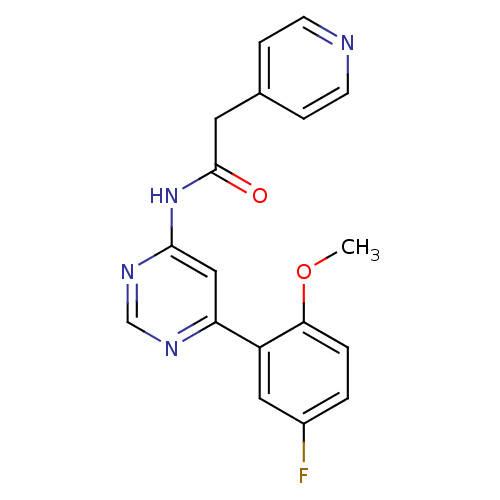
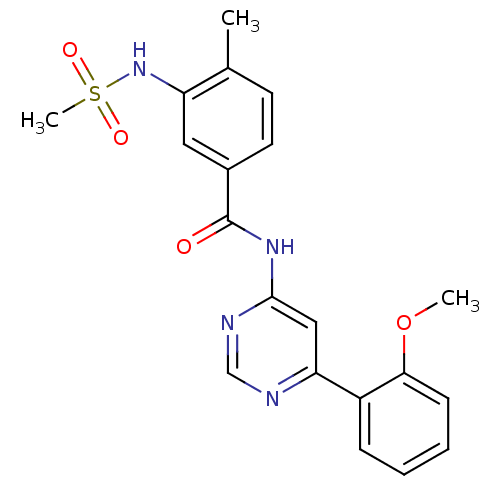
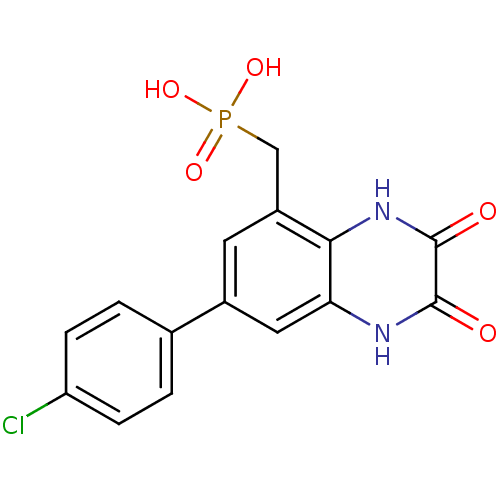
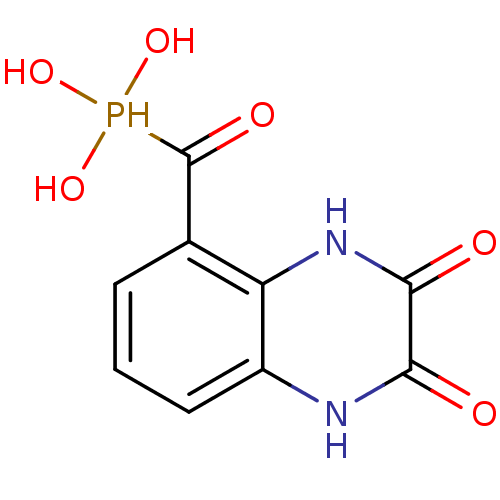
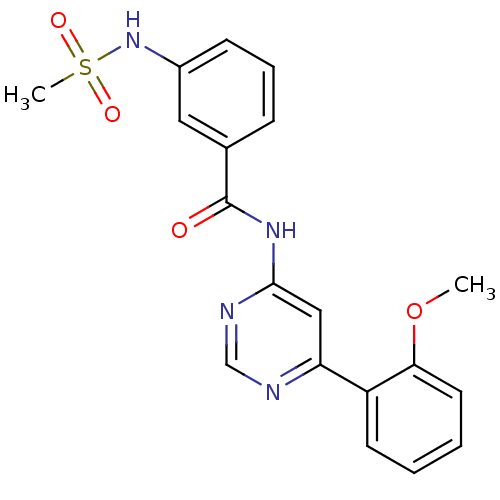
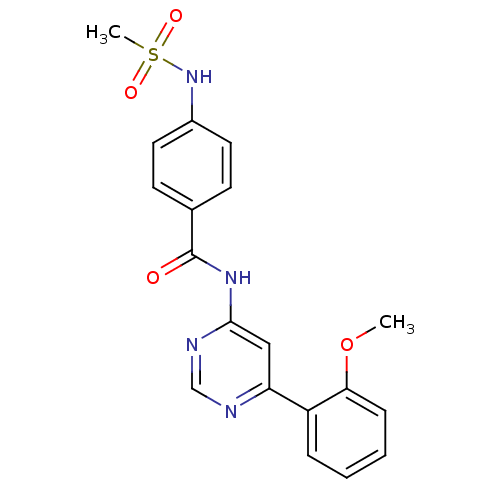
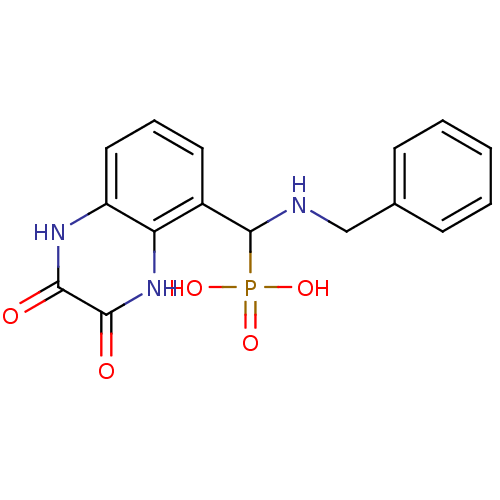
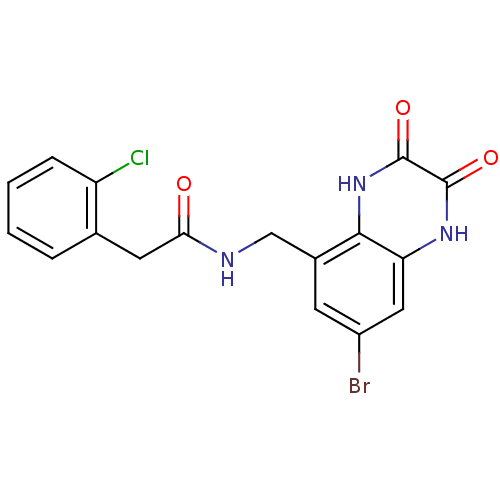
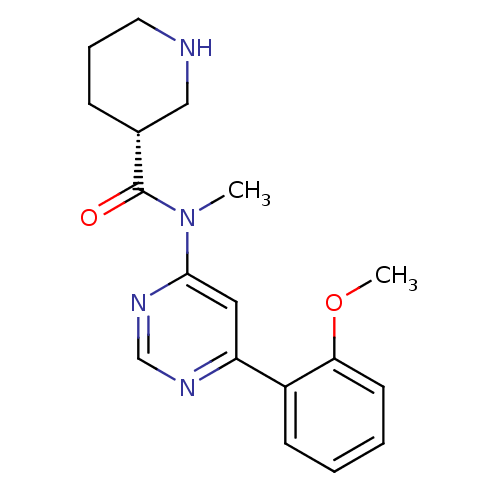
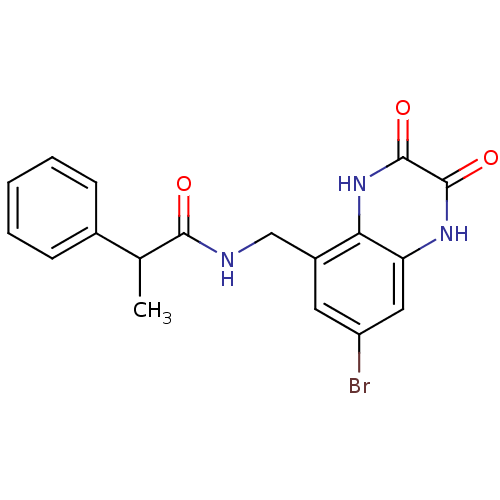
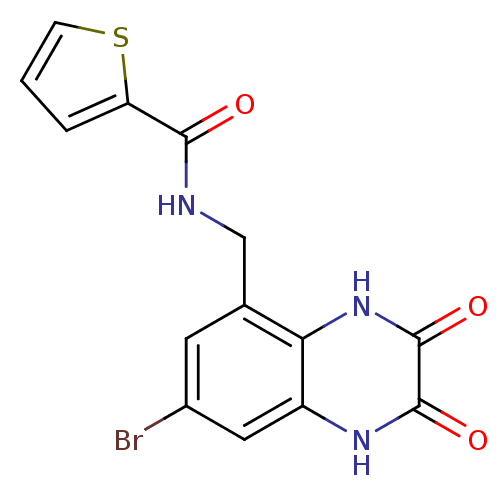
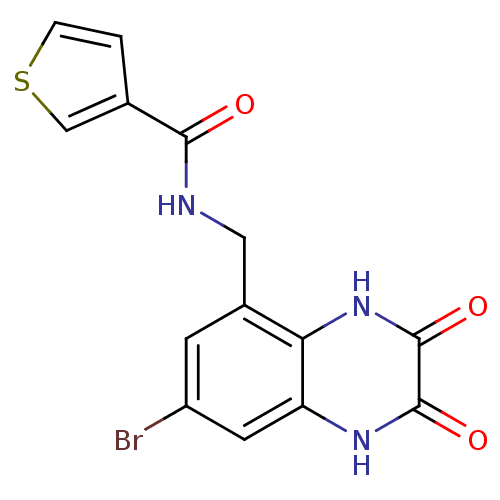
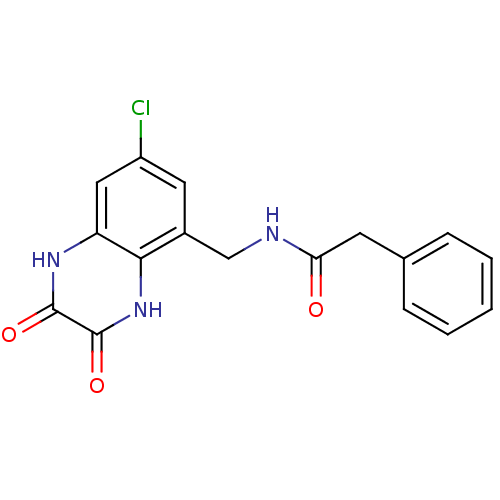
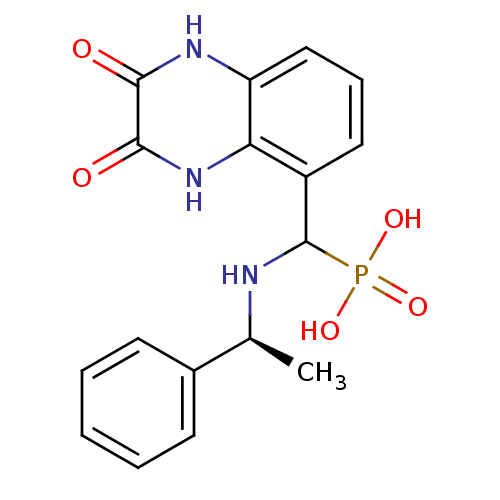
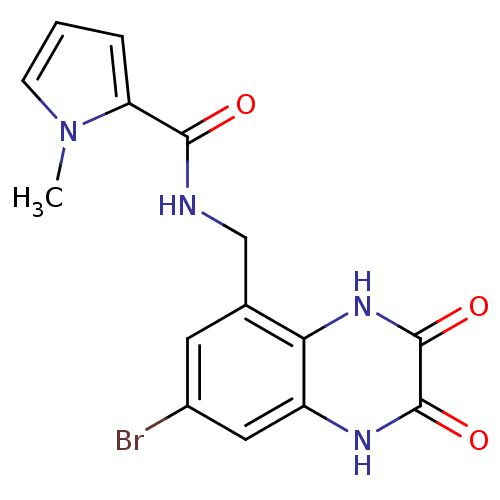
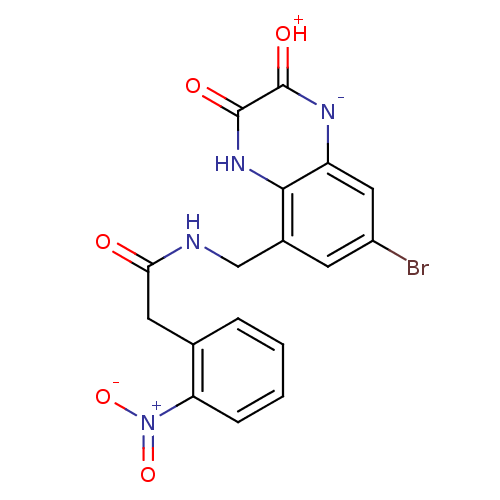
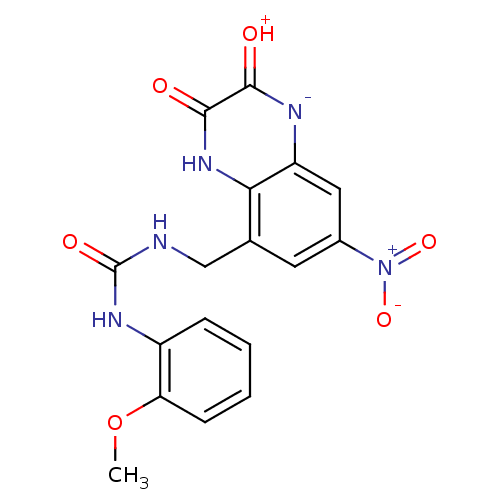
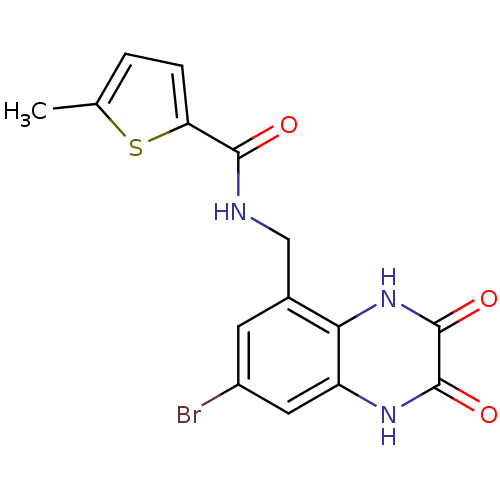
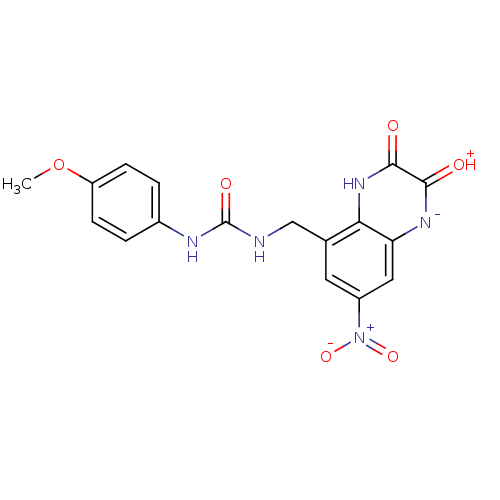
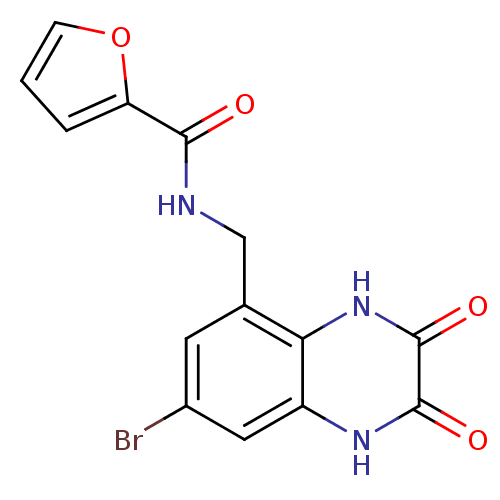
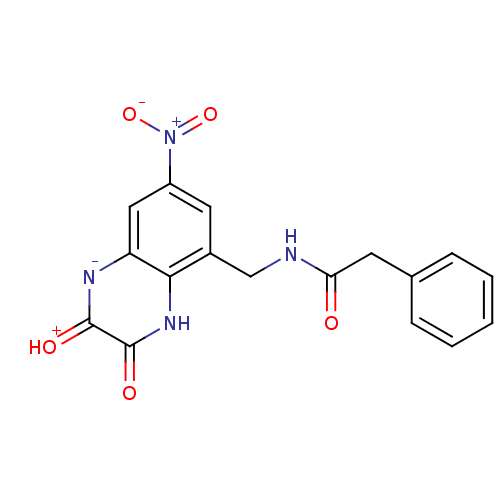
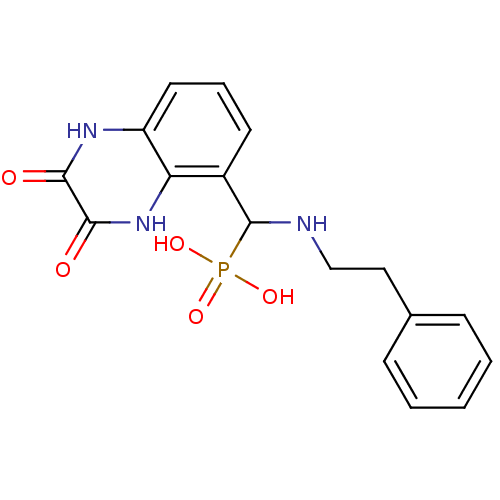
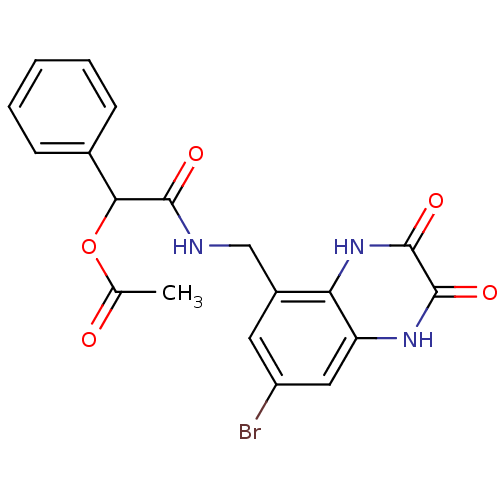
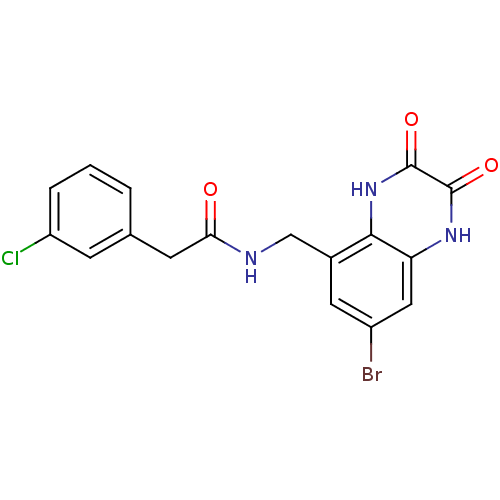
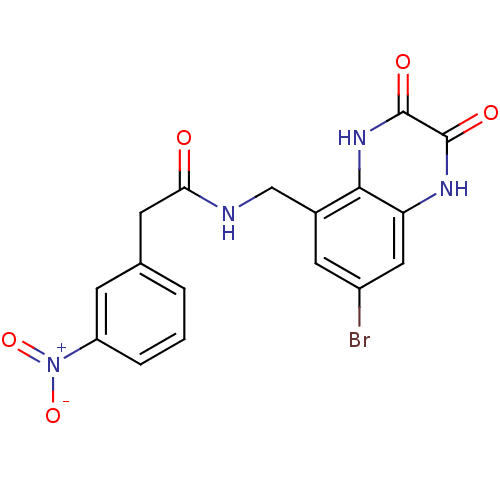
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 19nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Rattus norvegicus (Rat))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]ABP688 from mGluR5 in rat brain membraneMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Displacement of [3H]ABP688 from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.00500nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0150nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0180nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0190nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0290nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0390nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.116nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 0.173nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.08nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2A heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of glutamate-induced calcium releaseMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Activity at human recombinant mGluR5 expressed in L(tk-) cells assessed as inhibition of quisqualate-induced phosphoinositol accumulationMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 5(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Displacement of [3H]M-MPEP from human mGluR5 receptor expressed in L (tk-) cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.5Assay Description:All kinase assays were performed in 96-well FlashPlates from Perkin Elmer/NEN (Boston, Mass., USA) in a 50 μl reaction volume. The reaction mixt...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro binding assay for the displacement of [3H]MDL-105519 from the glycine-site of NMDA receptorsMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)