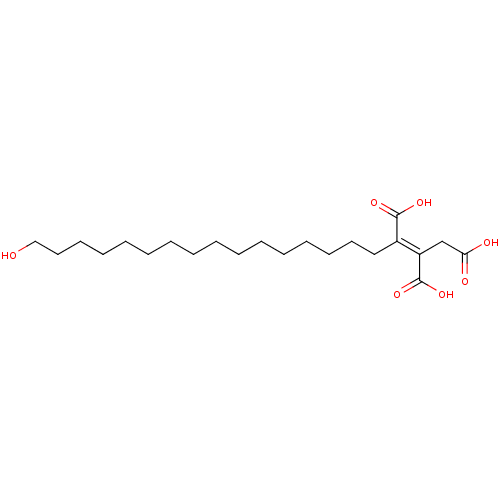
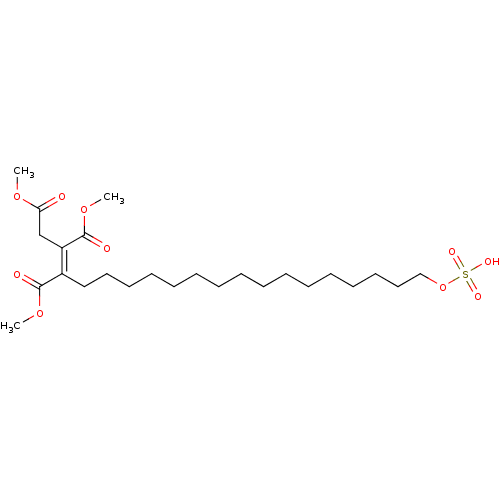
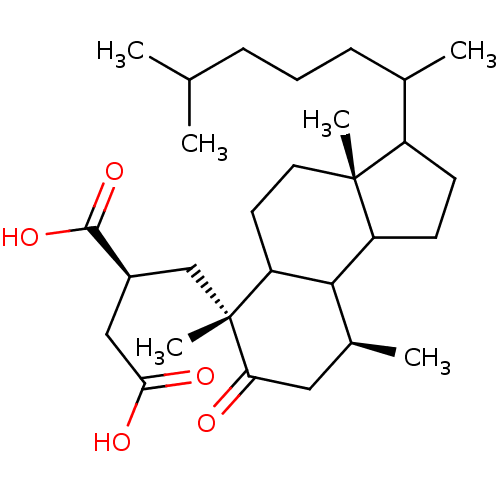
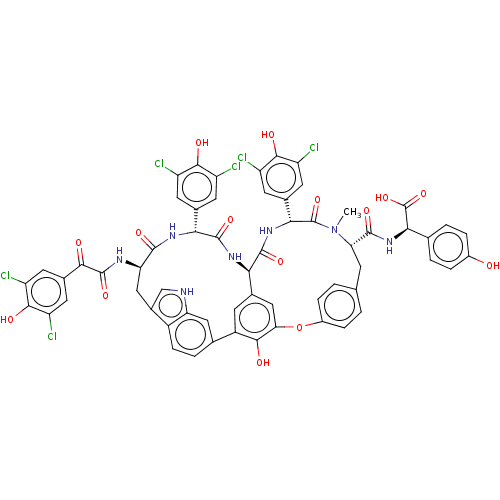
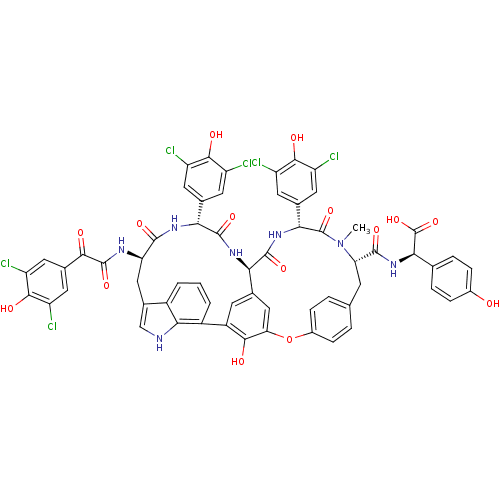
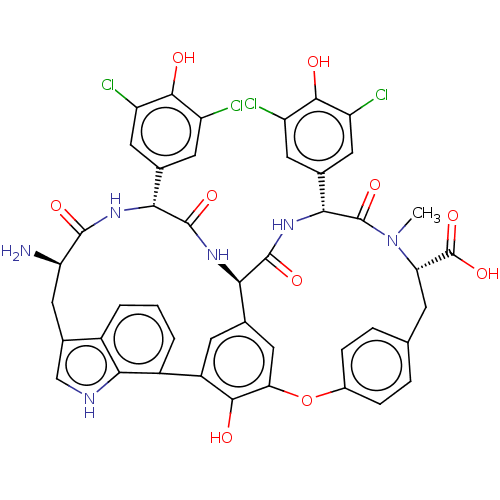
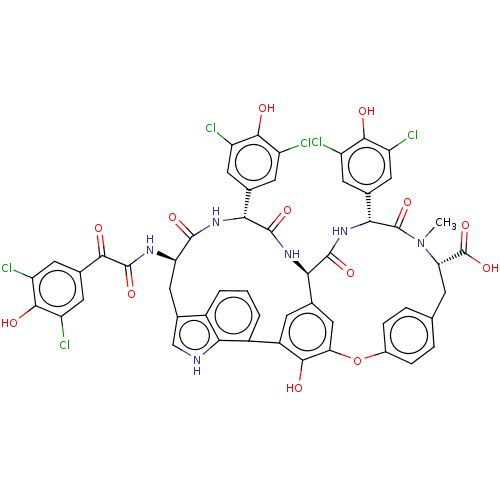
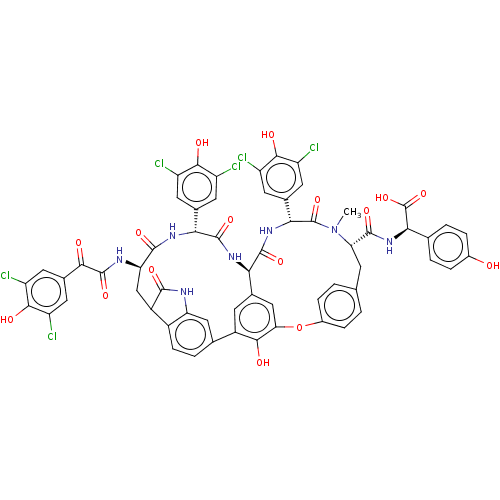
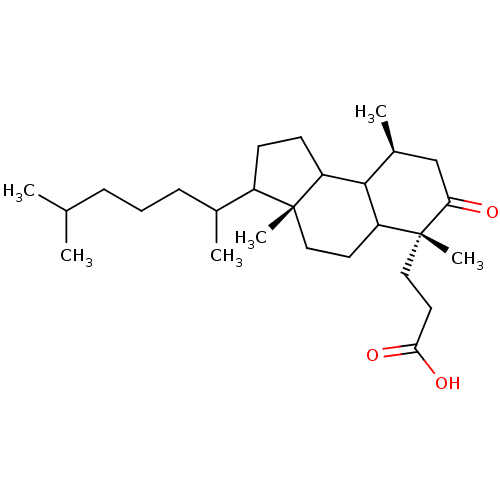
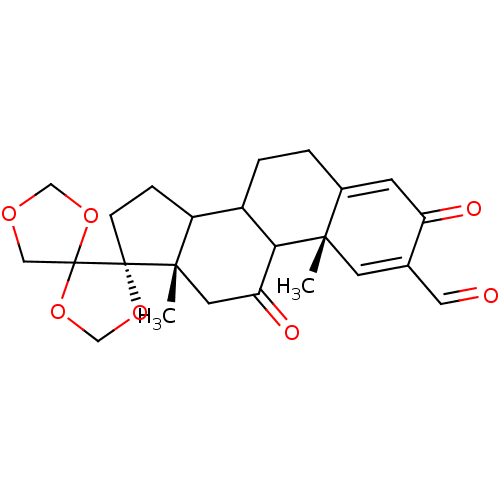
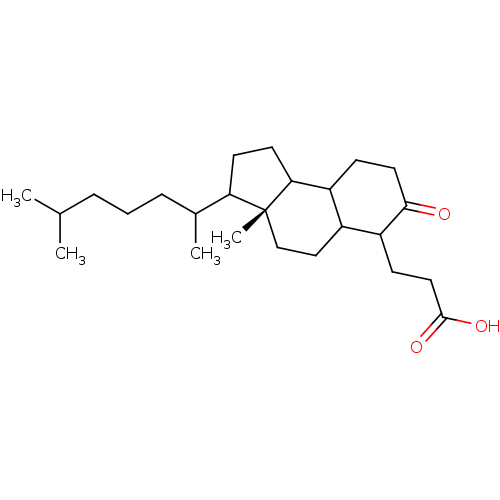
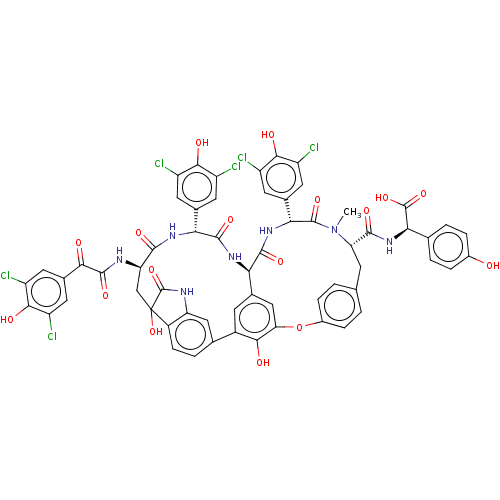
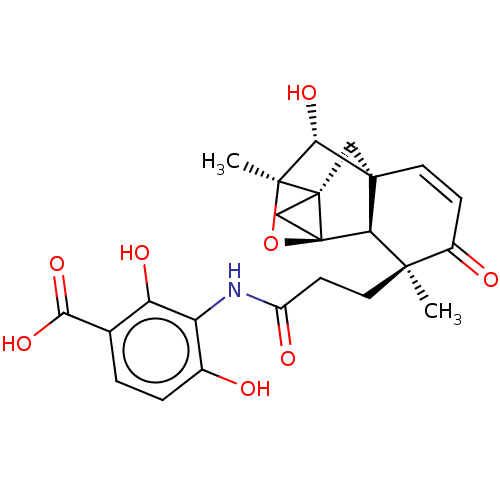
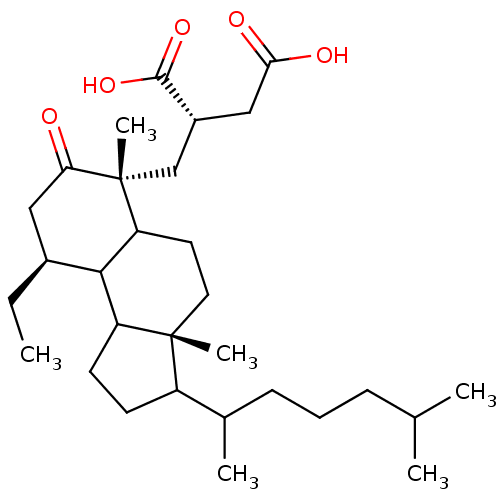
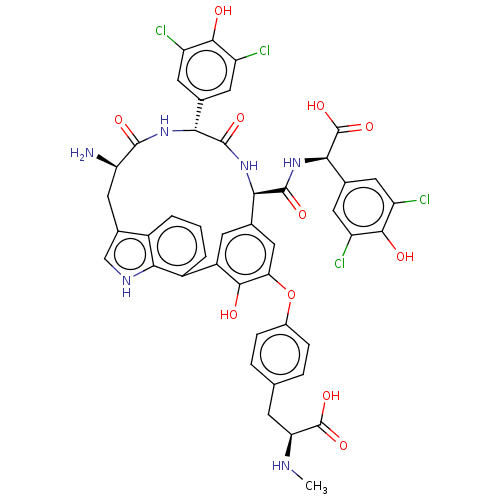
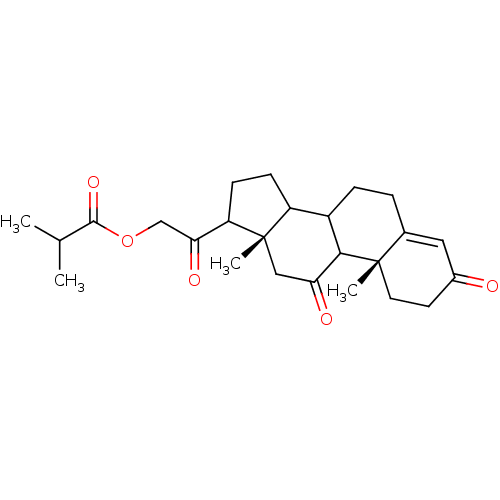
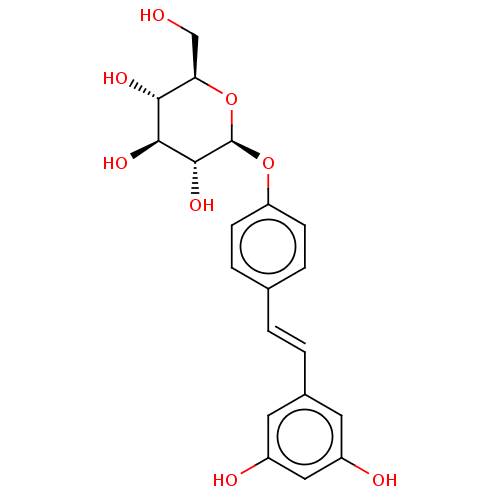
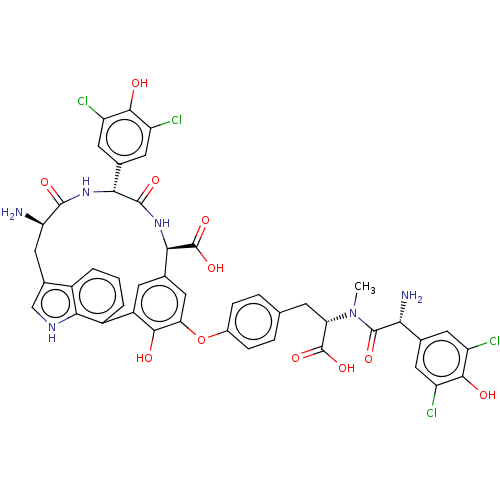
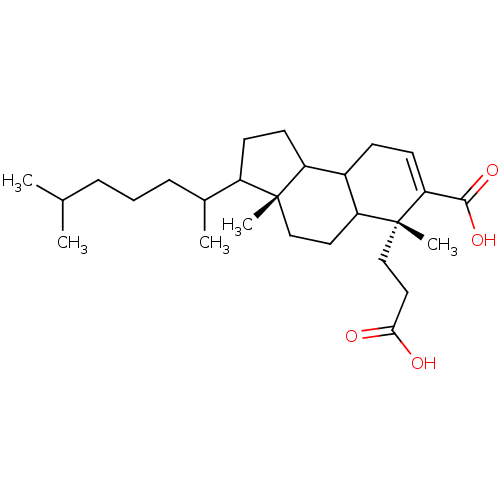
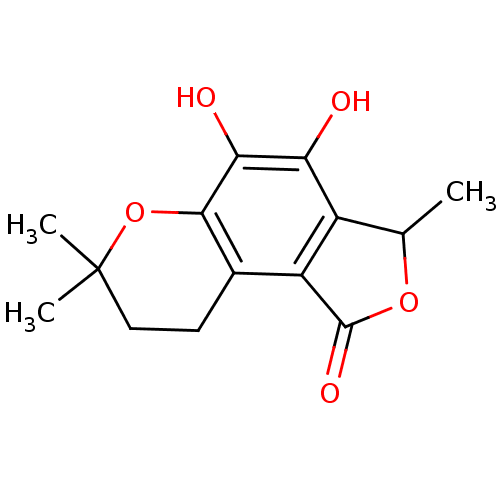
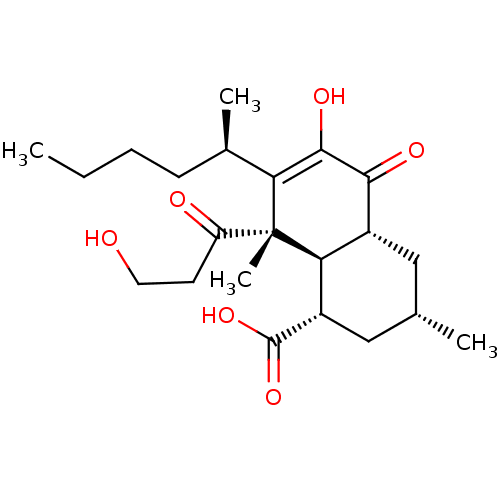
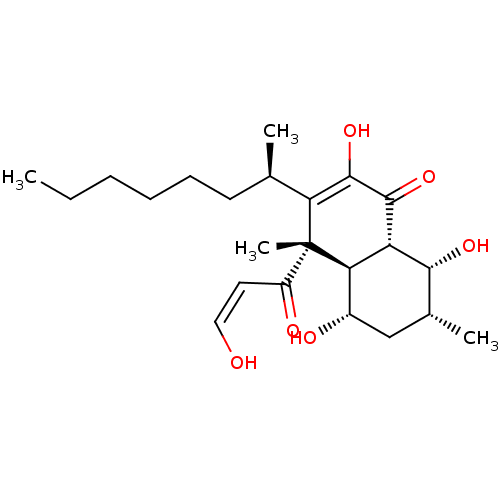
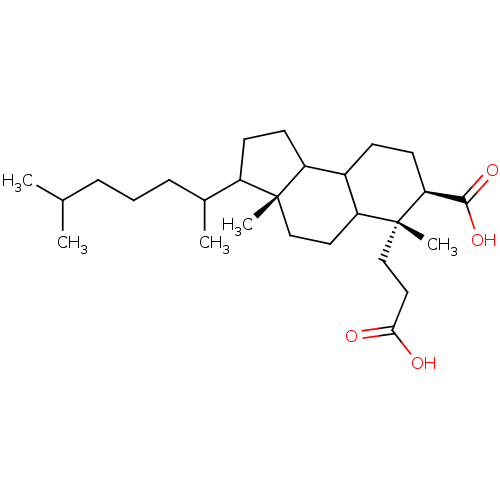
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataKi: 4.5nMAssay Description:In vitro inhibition of human recombinant Protein farnesyltransferase with respect to FPPMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:In vitro inhibition of human recombinant Protein farnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:In vitro inhibition of human recombinant Protein farnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:In vitro inhibition of human recombinant Protein farnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of human Farnesyltransferase using Ras-CVLSMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of disintegration activity of HIV1 intact integraseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of disintegration activity of HIV1 integrase catalytic core domain (50 to 212)More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Displacement of [125I]gp-120 from human CCR5 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human Farnesyltransferase using Ras-CVIMMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [3H2]F3-methyl AA from LXRalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [3H2]F3-methyl AA from LXRbeta by scintillation proximity assayMore data for this Ligand-Target Pair
Target3-oxoacyl-[acyl-carrier-protein] synthase 2(Staphylococcus aureus)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.85E+3nMAssay Description:Inhibition of Staphylococcus aureus FASF assessed as [2-14C]-malonyl CoA incorporation after 90 mins by cell free-based scintillation countingMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of integration activity of HIV1 intact integraseMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Displacement of [3H2]F3-methylAA from human LXRalpha expressed in Escherichia coli BL21 cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 6.80E+3nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.68E+3nMAssay Description:Inhibition of rat brain PKCMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of HIV1 integrase 3' processing/strand transfer coupled activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Displacement of [3H2]F3-methyl AA from LXRalpha by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of human Farnesyltransferase using Ras-CVLSMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Displacement of [3H2]F3-methylAA from human LXRbeta expressed in Escherichia coli BL21 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Displacement of [3H2]F3-methyl AA from LXRbeta by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of rat brain PKCMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of MIP1alpha binding to human CCR5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibitory activity against human FarnesyltransferaseMore data for this Ligand-Target Pair