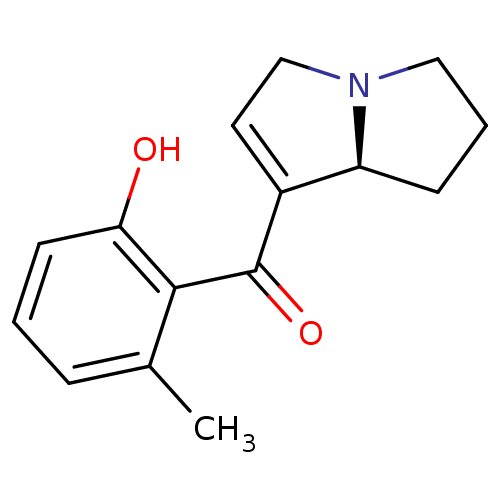
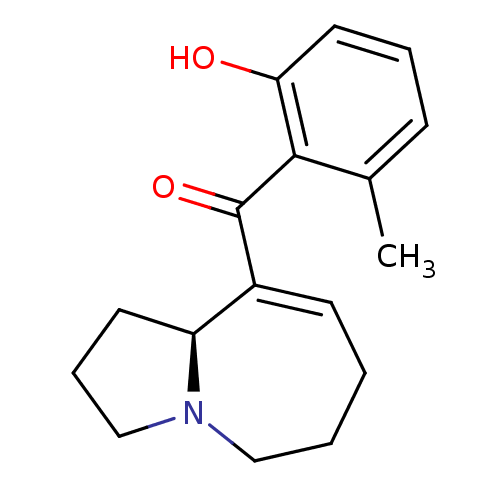
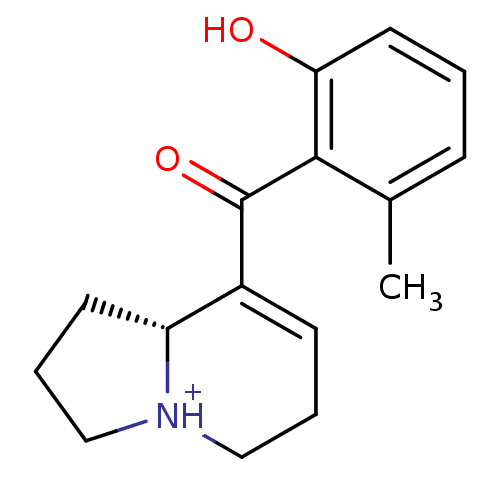
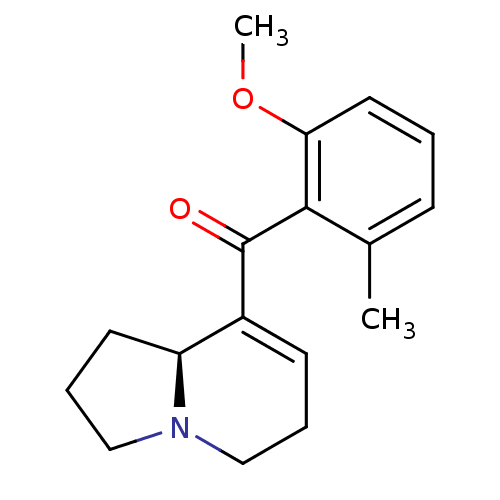
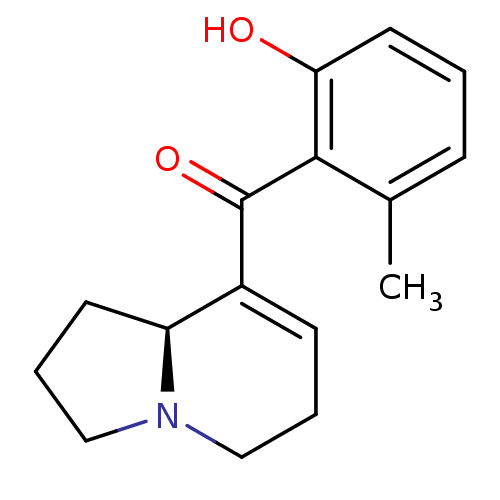
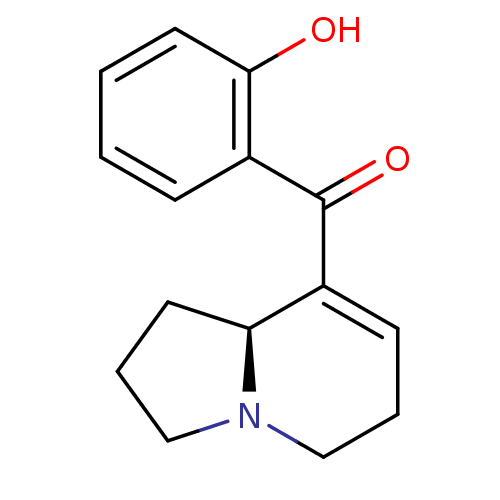
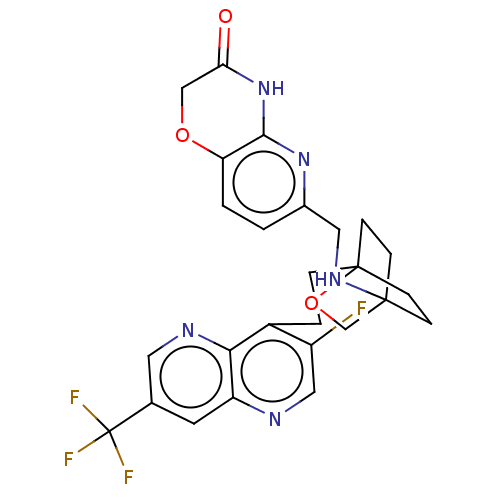
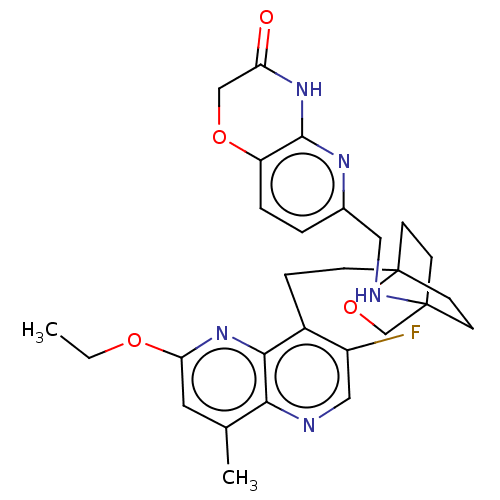
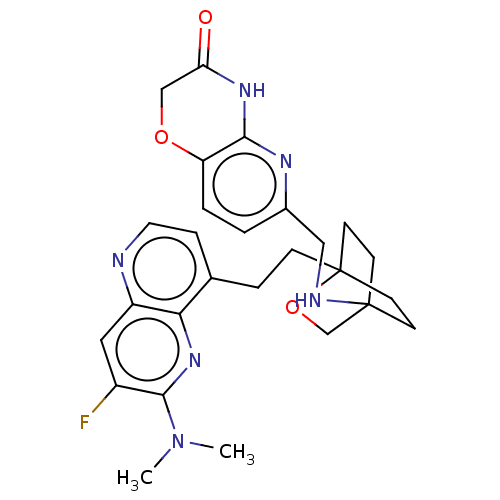
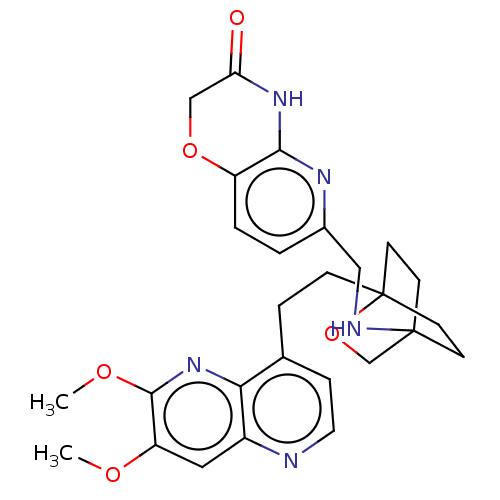
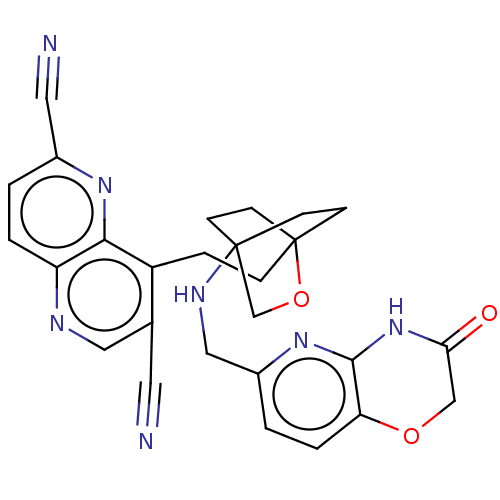
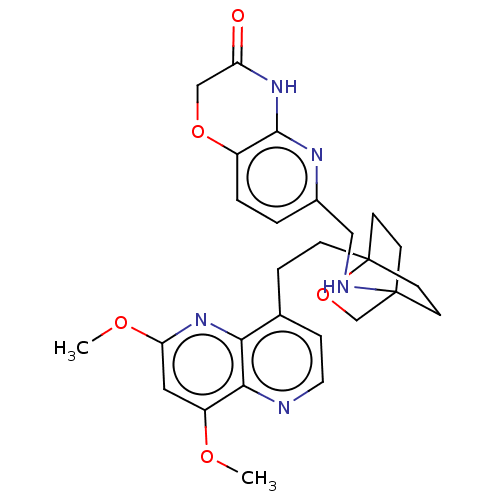
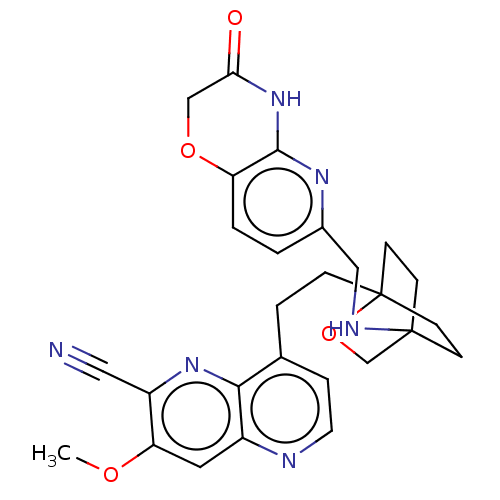
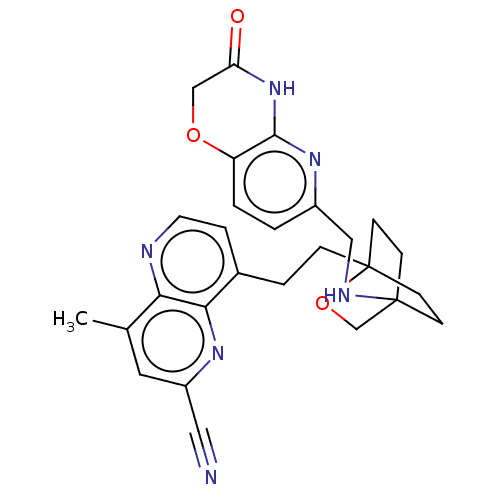
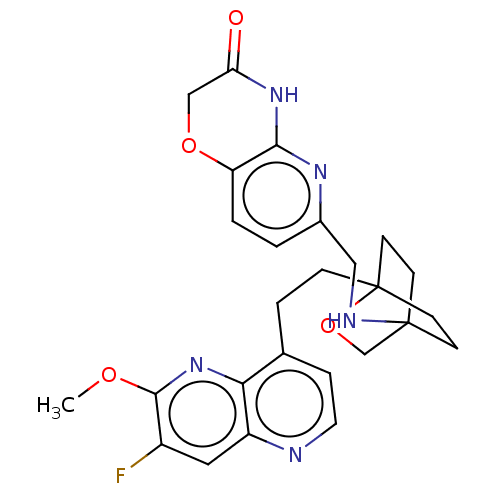
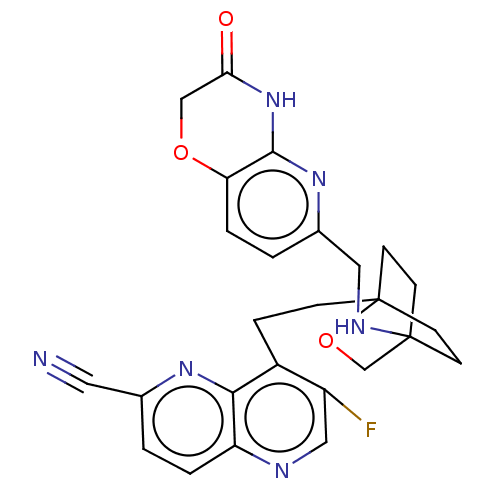
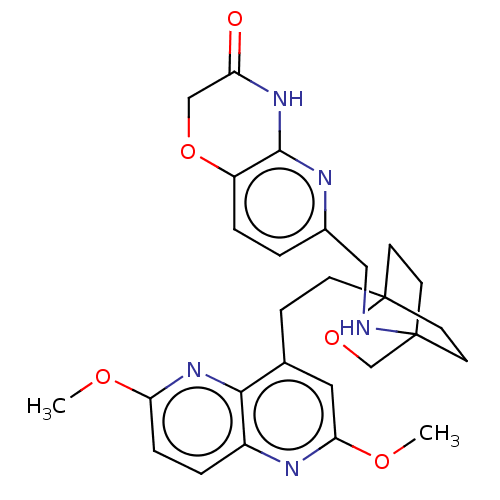
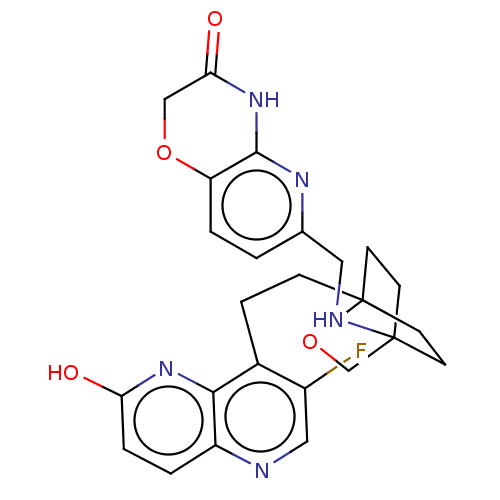
Affinity DataKi: 3.20E+3nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+4nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 2.40E+4nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+4nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.90E+4nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 8.30E+4nMAssay Description:Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: 9.40E+4nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.390nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.870nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.890nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.990nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.13nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate after 60 mins by OPA reagent based fluorescence assayMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetUDP-3-O-acyl-N-acetylglucosamine deacetylase(Escherichia coli)
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysisMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.70nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Inhibition of MK499 binding to human ERGMore data for this Ligand-Target Pair