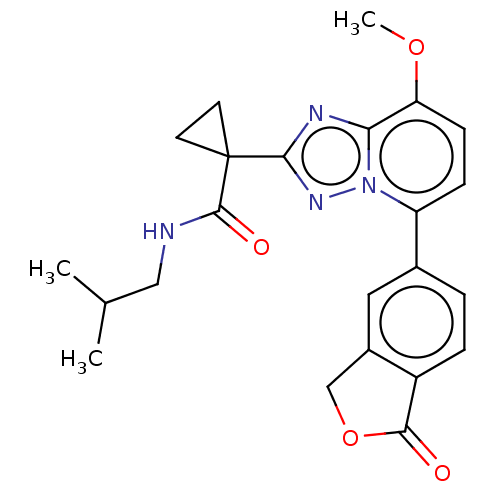
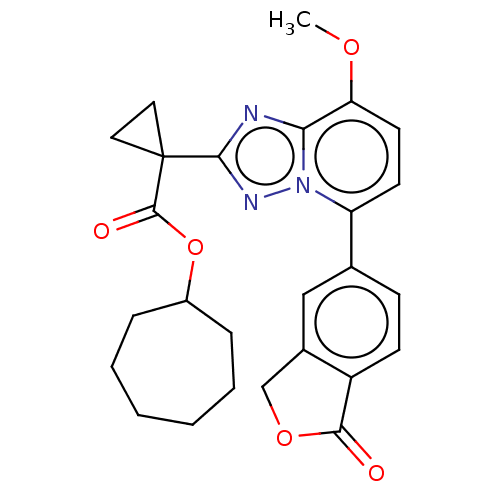
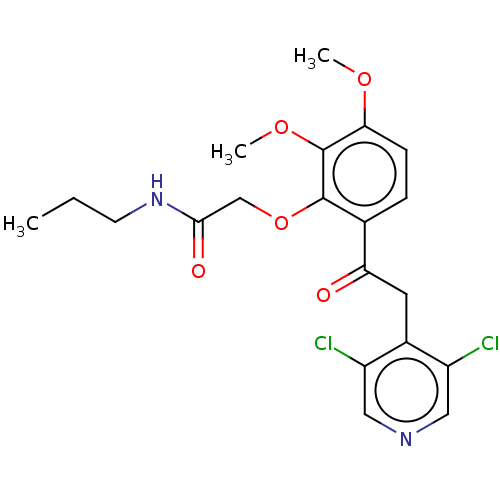
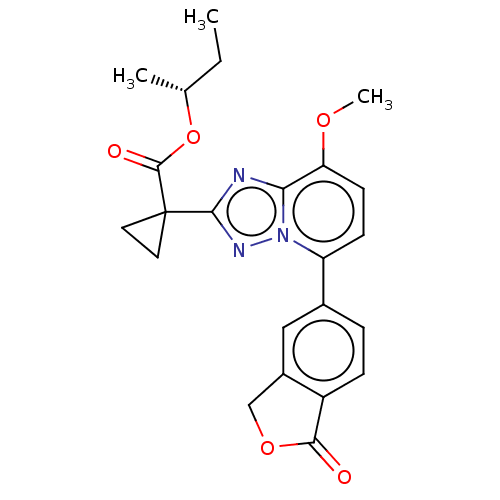
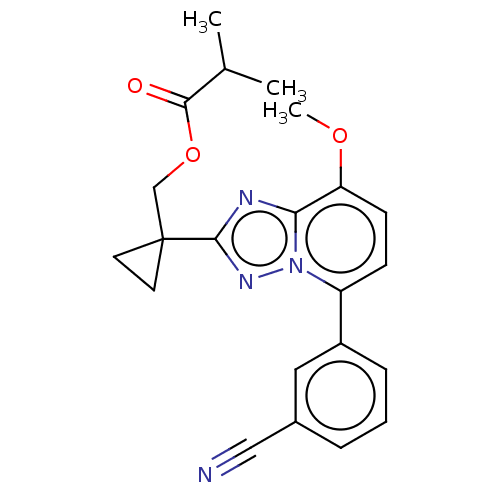
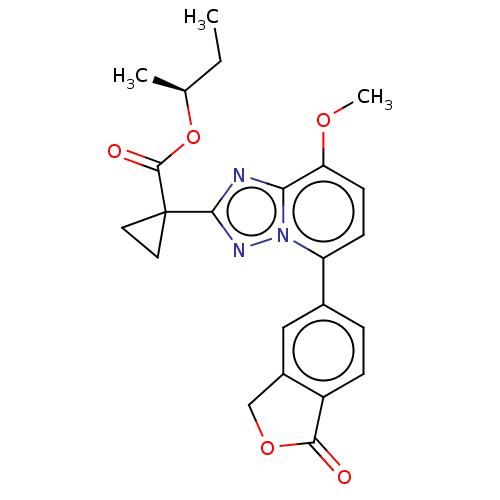
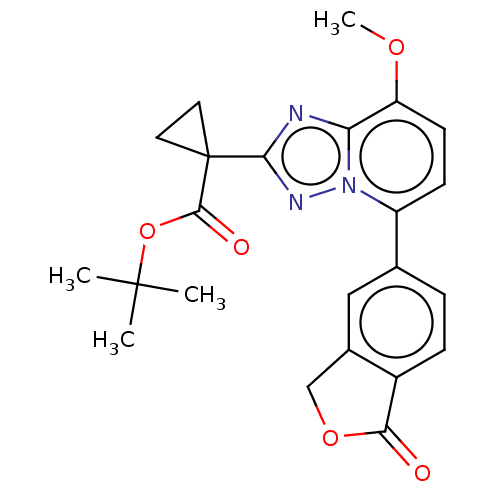
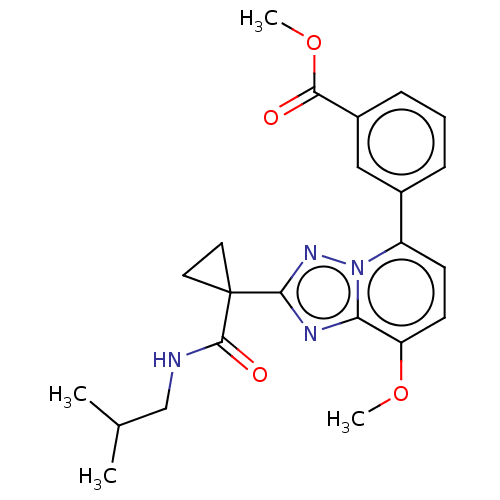
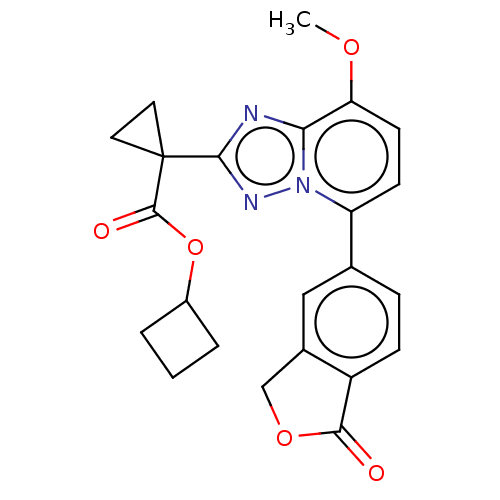
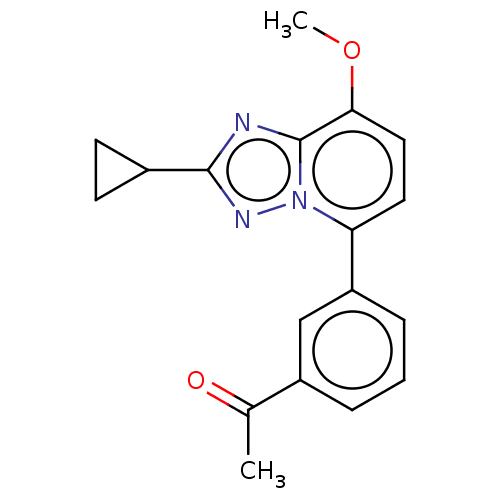
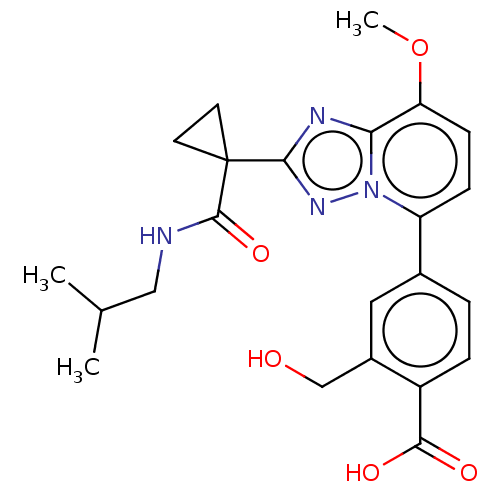
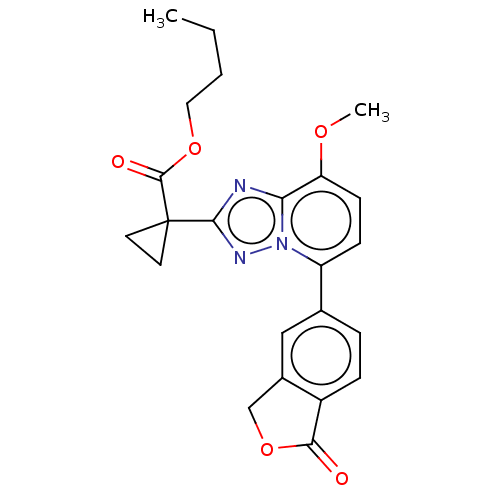
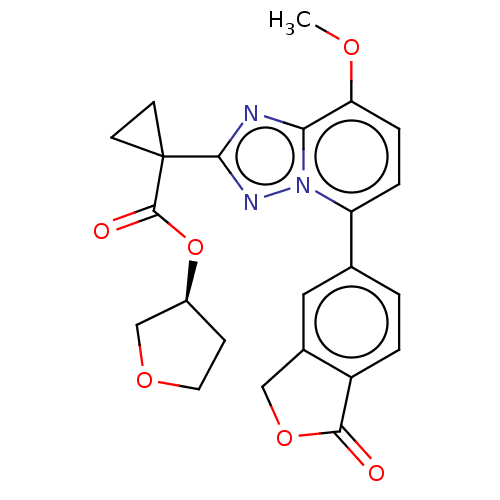
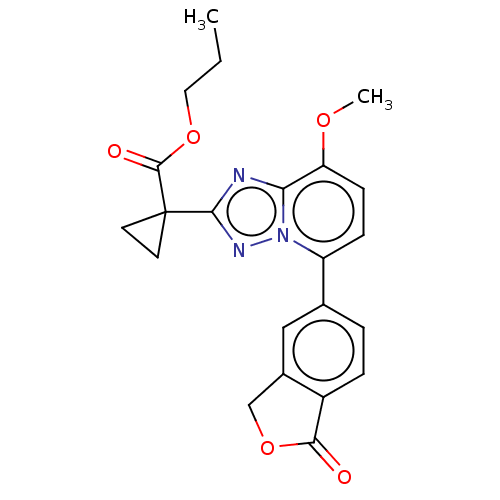
Affinity DataKi: 0.200nM ΔG°: -54.8kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -54.8kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
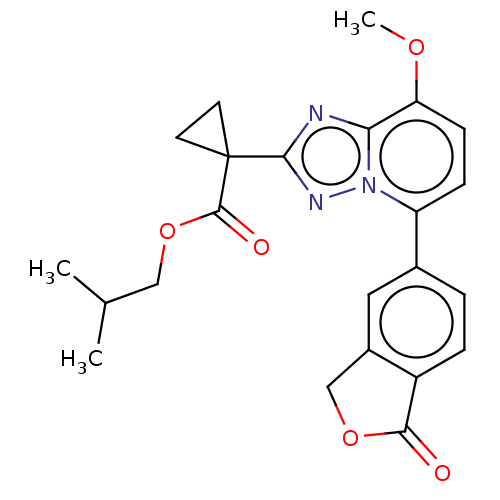
Affinity DataKi: 0.320nM ΔG°: -53.6kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
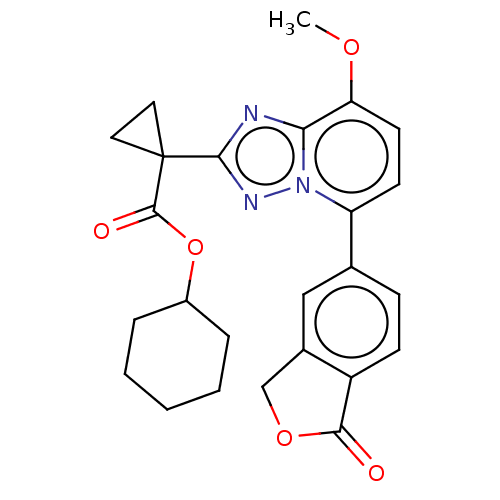
Affinity DataKi: 0.790nM ΔG°: -51.4kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
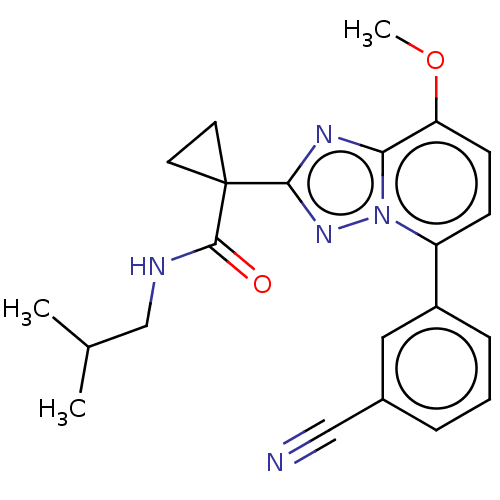
Affinity DataKi: 1.20nM ΔG°: -50.4kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 2nM ΔG°: -49.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 3nM ΔG°: -48.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -43.0kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 40nM ΔG°: -41.8kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 398nM ΔG°: -36.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nM ΔG°: -33.3kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nM ΔG°: -32.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 5.01E+3nM ΔG°: -29.9kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of PDE4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human PDE4A catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human PDE4B catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of PDE4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of PDE4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human PDE4C catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.20nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human PDE4D catalytic domain using cAMP and FAM-conjugated cAMP by IMAP FRET progressive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of PDE4D in human PBMC assessed as reduction in lipopolysaccharide-induced TNFalpha production pre-incubated for 30 mins before LPS stimul...More data for this Ligand-Target Pair

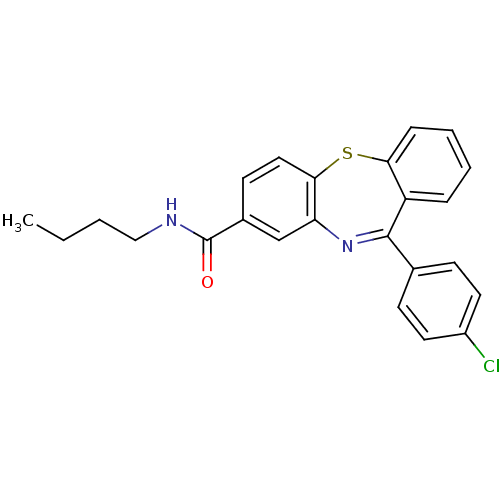

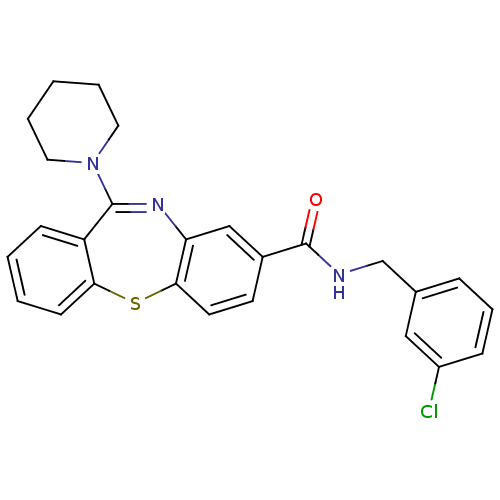
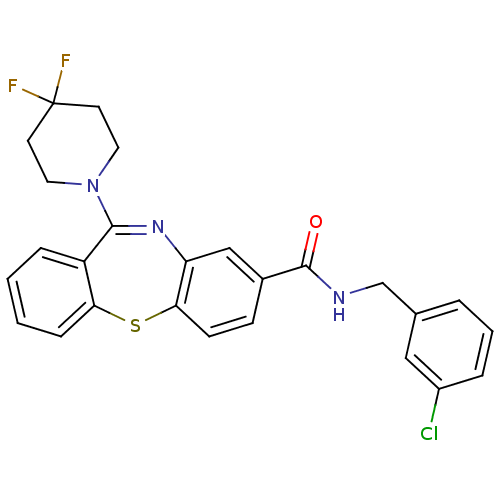
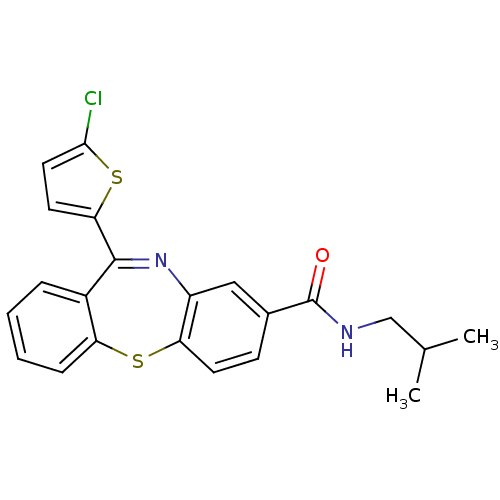
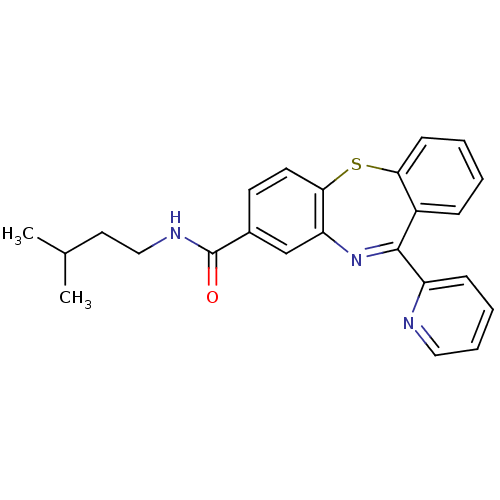

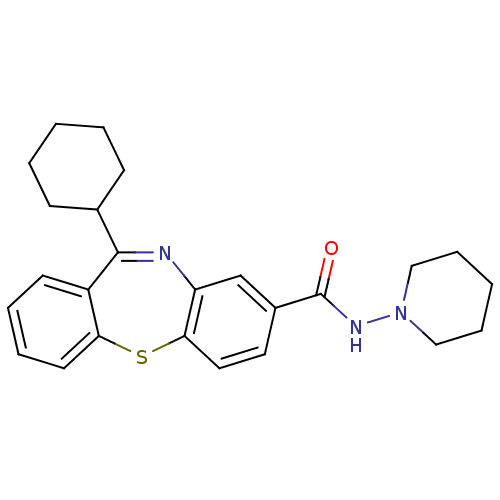
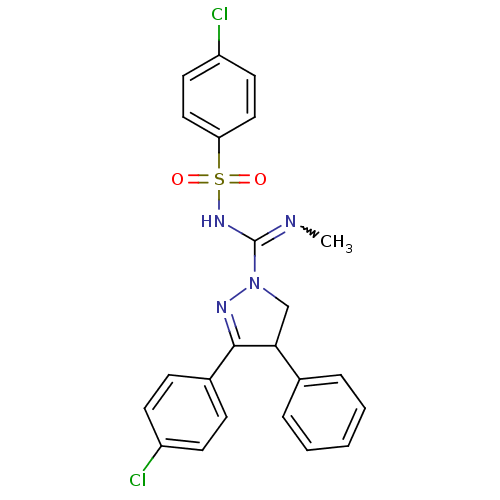
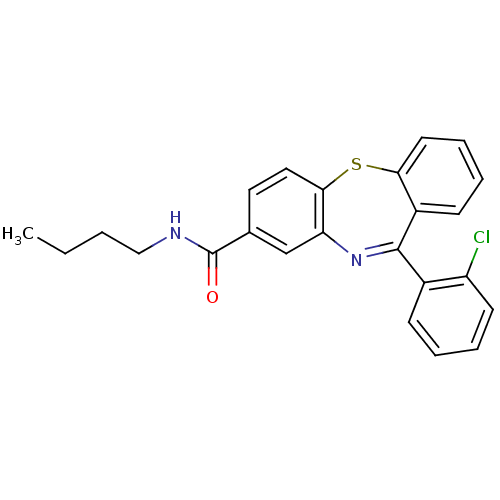
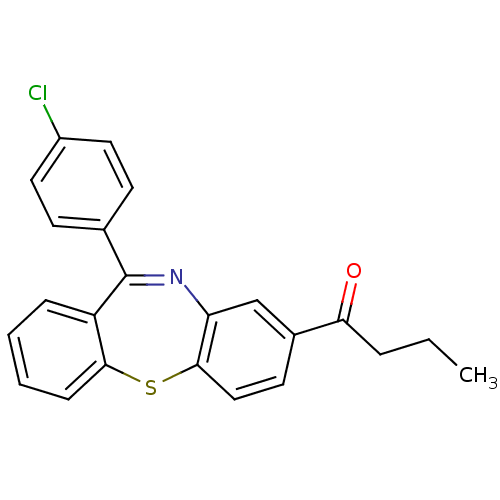

 3D Structure (crystal)
3D Structure (crystal)