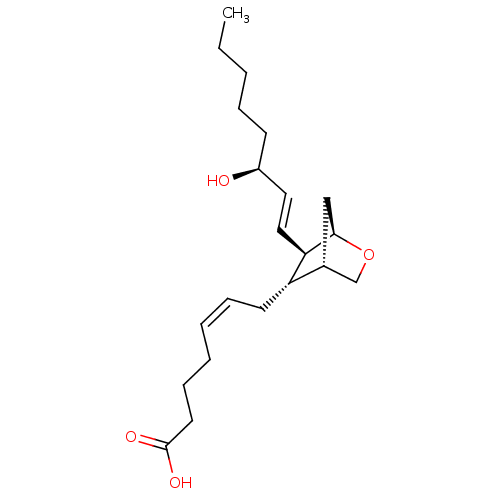
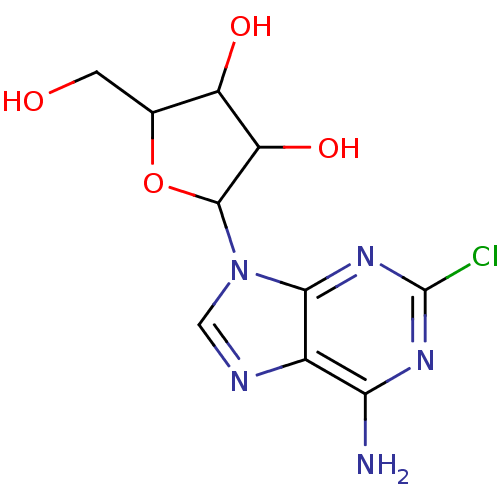
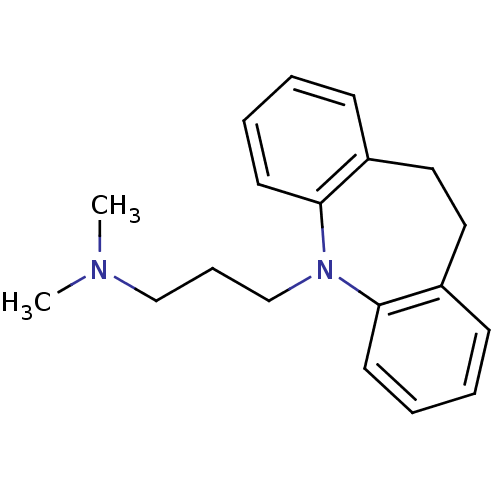
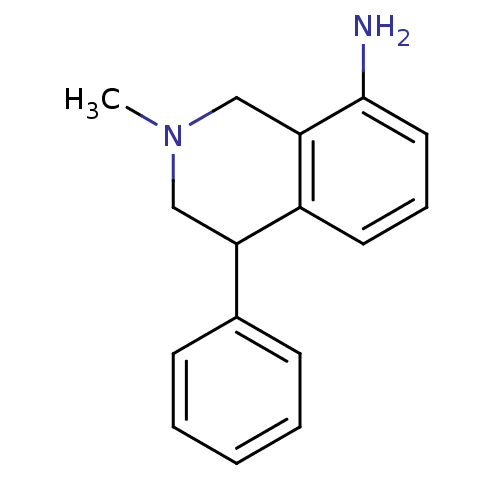
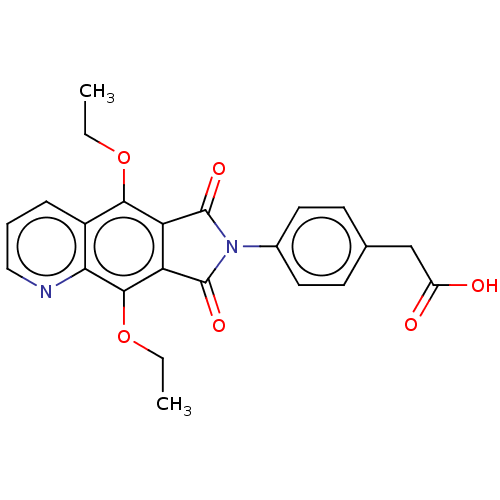
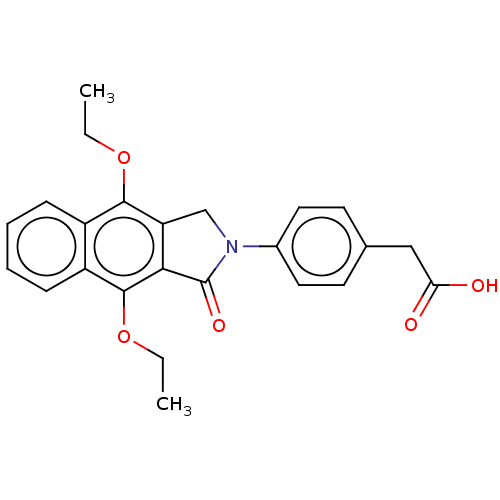
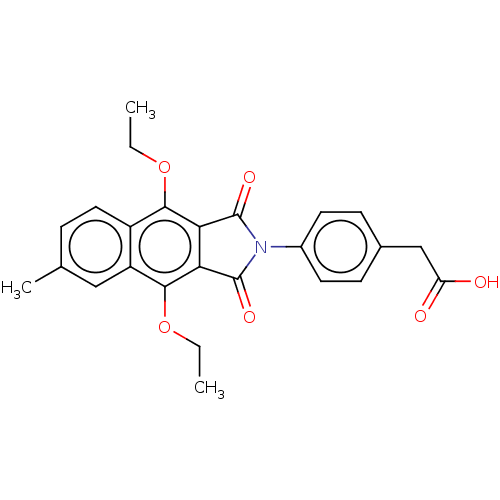
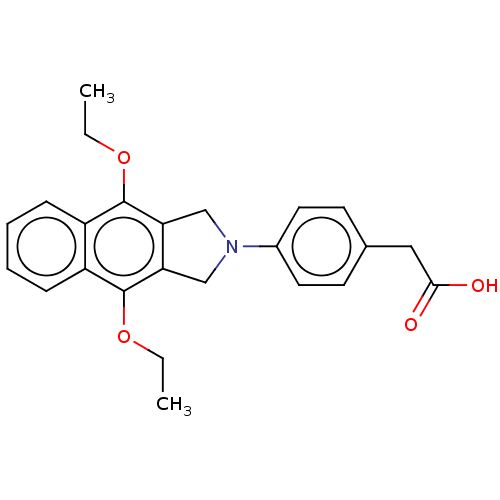
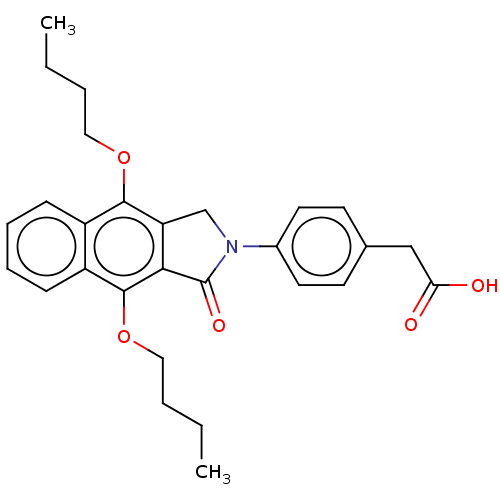
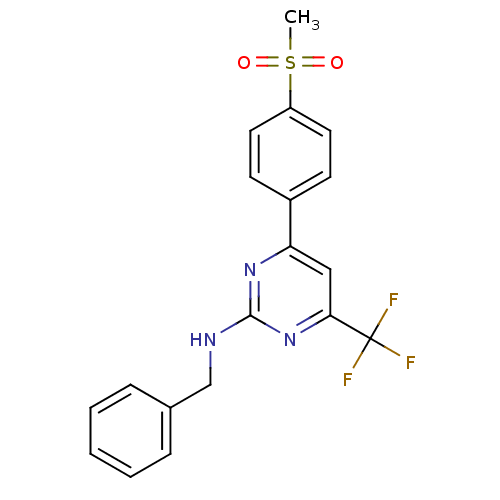
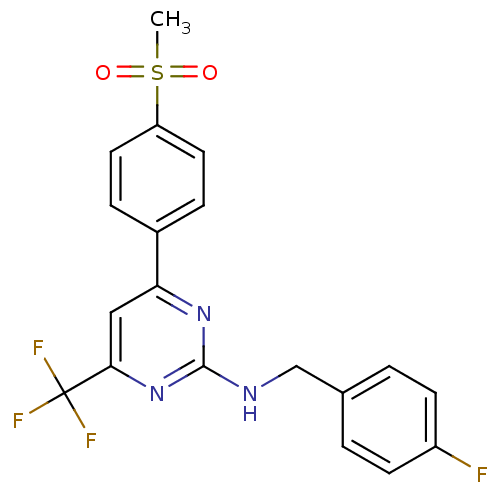
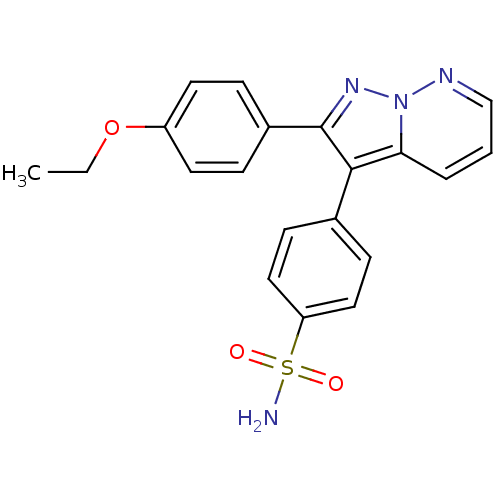
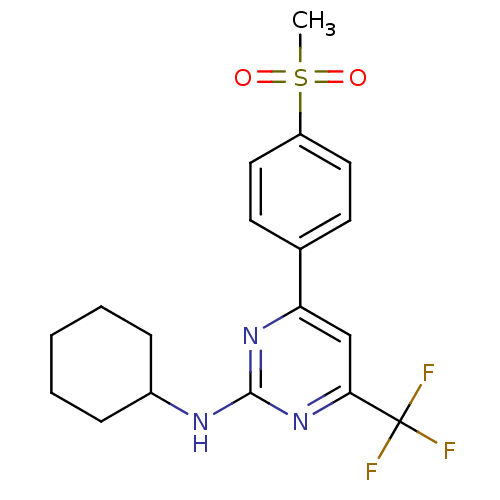
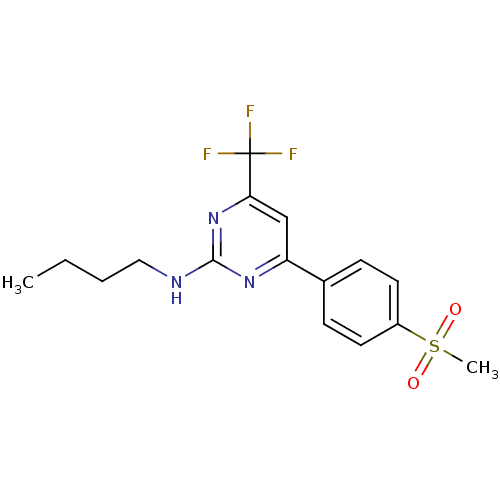
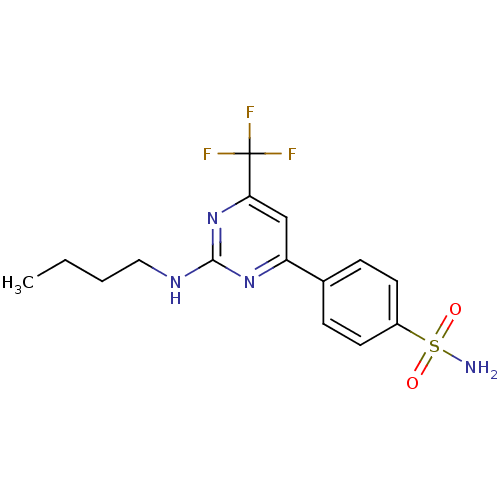
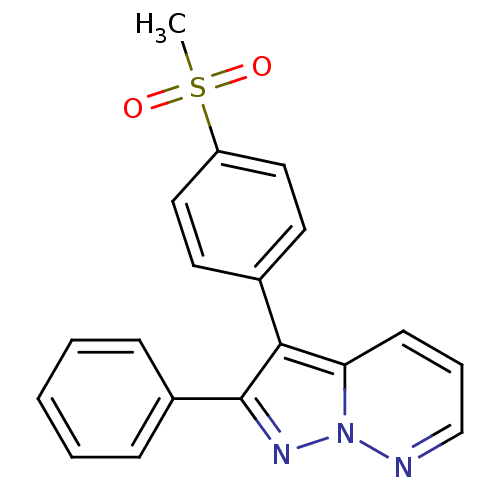
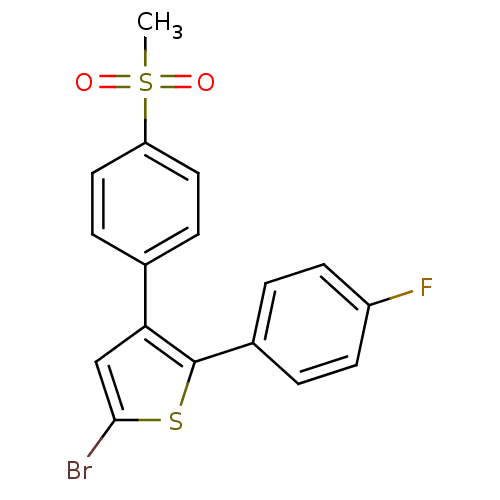
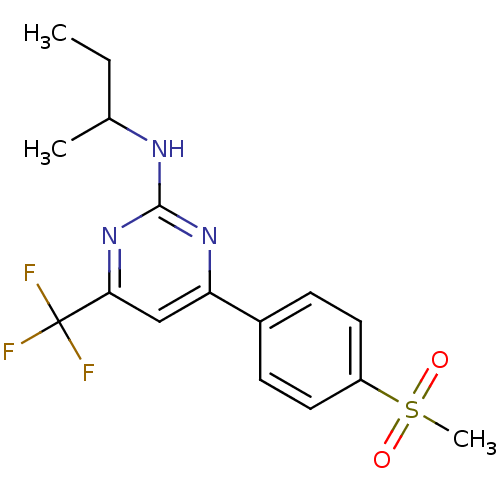
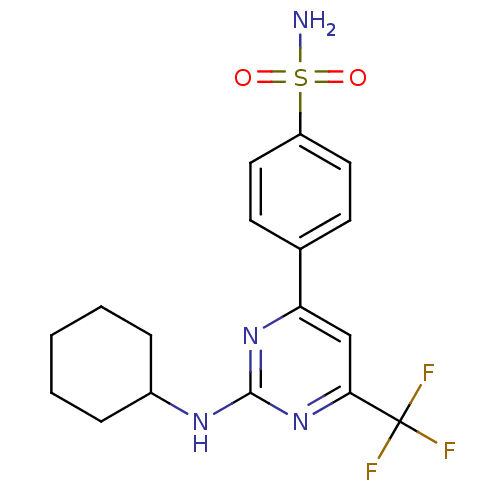
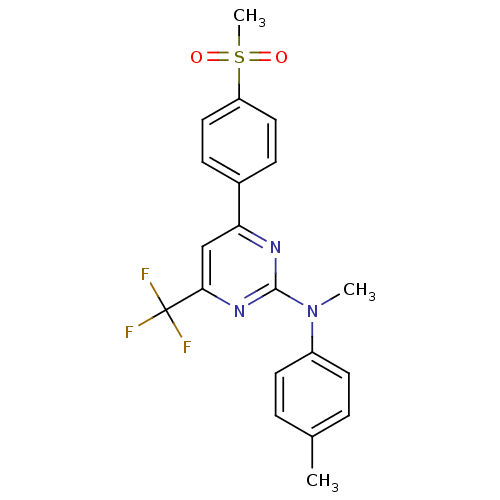
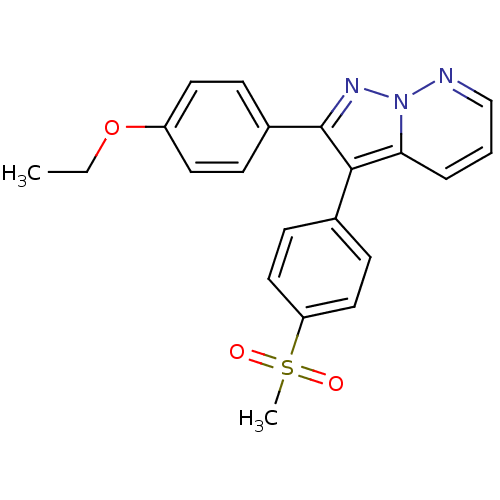
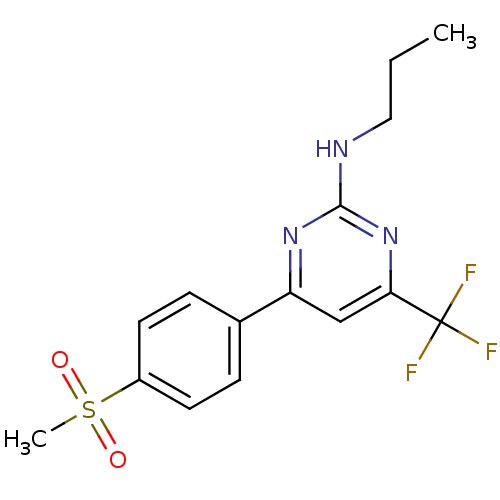
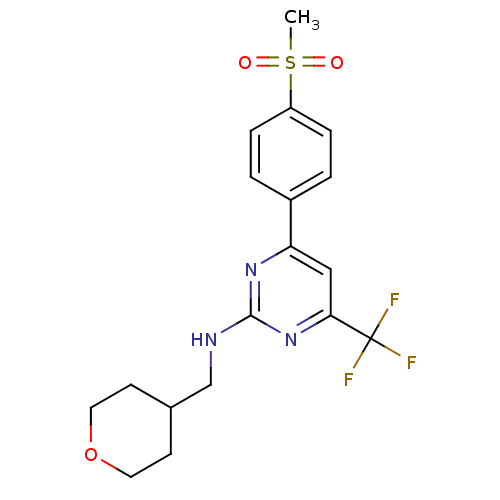
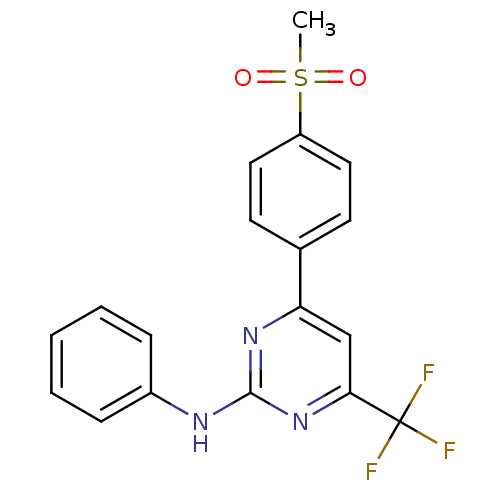
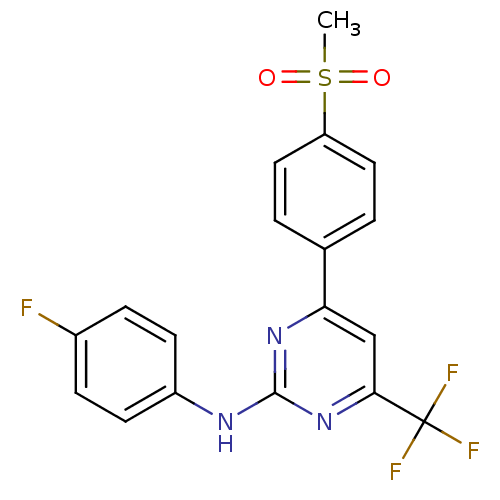
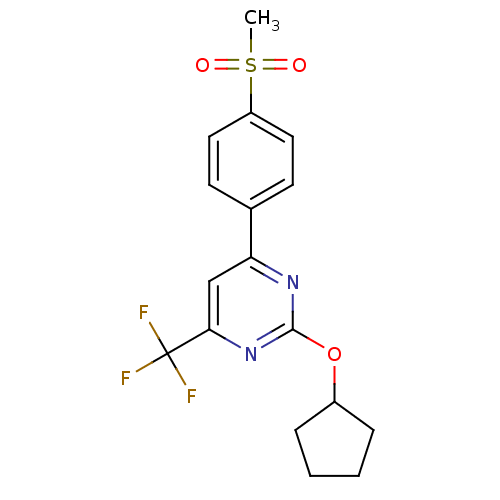
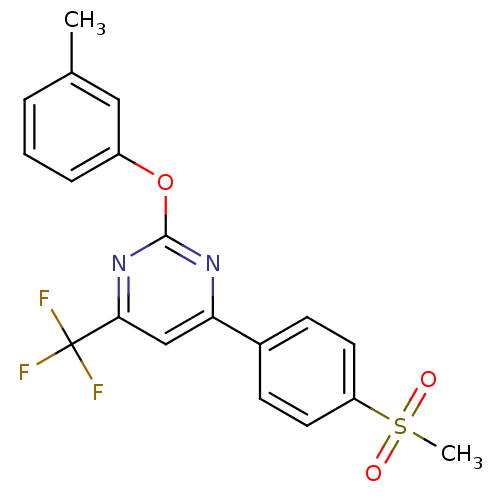
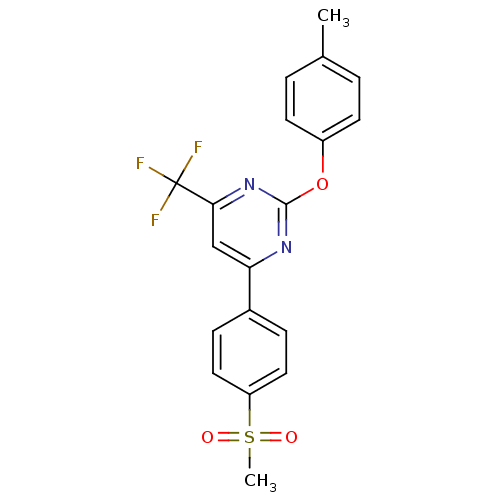
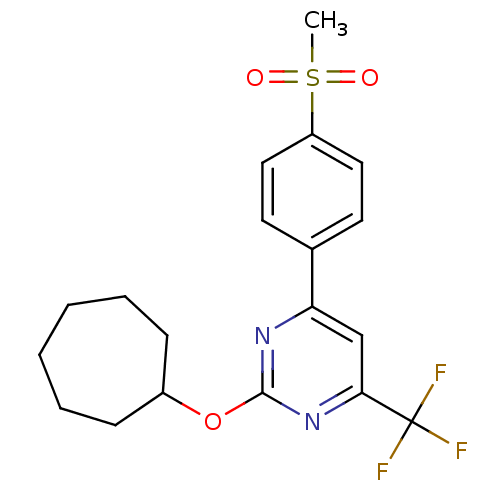
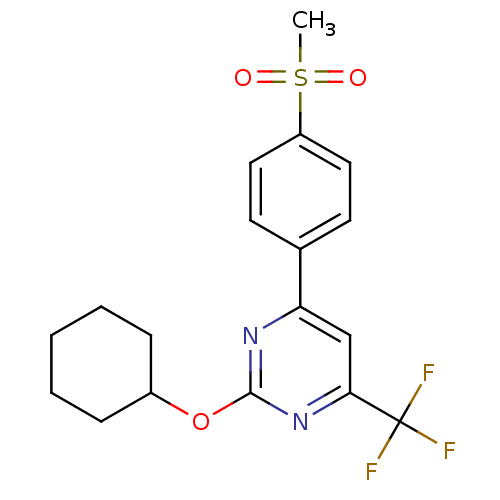
Affinity DataKi: 0.100nMAssay Description:Displacement of [3H]sulpiride from dopamine D2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]U-69593 from kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]pirenzepine from muscarinic M1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]AF-DX384 from muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]LTD4 from LTD4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]pyrilamine from histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Displacement of [3H]DAGO from mu opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Displacement of [3H]RX781094 from alpha2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Displacement of [3H]LTB4 from LTB4RMore data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]SQ29548 from thromboxane A2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [3H]CPX from adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]citalopram from serotonin transporterMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of [3H]WIN-from cocaine site of dopamine transporterMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Displacement of [3H]SCH23390 from dopamine D1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Displacement of [3H]DPDPE from delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 158nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 158nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 398nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 580nMAssay Description:Displacement of [3H]DMI from norepinephrine transporterMore data for this Ligand-Target Pair
Affinity DataKi: 631nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 631nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: <6.31E+3nMAssay Description:Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibition of human cyclooxygenase-2 expressed in COS cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human cyclooxygenase-2 expressed in COS cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human cyclooxygenase-2 expressed in COS cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of COX2 in human whole blood assessed as inhibition of lipopolysaccharide-stimulated PGE2 production after 24 hrs by enzyme immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in baculovirus-infected SF9 cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production treated 1 ...More data for this Ligand-Target Pair




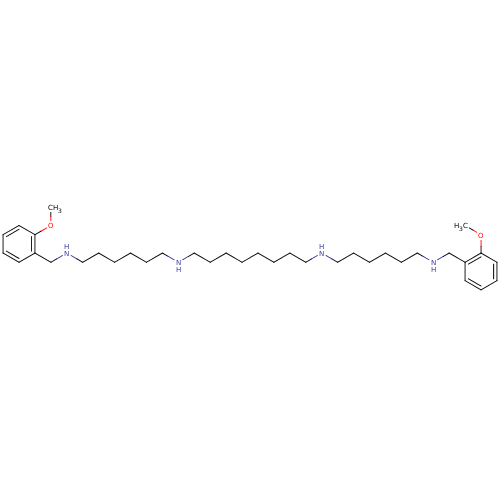
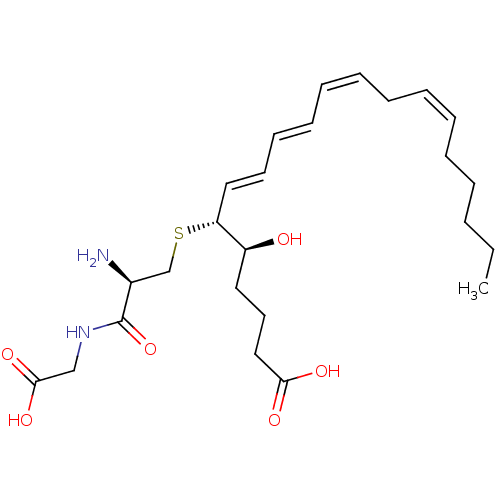

 3D Structure (crystal)
3D Structure (crystal)