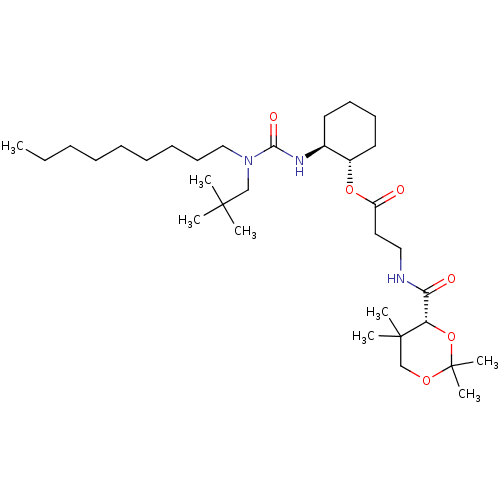
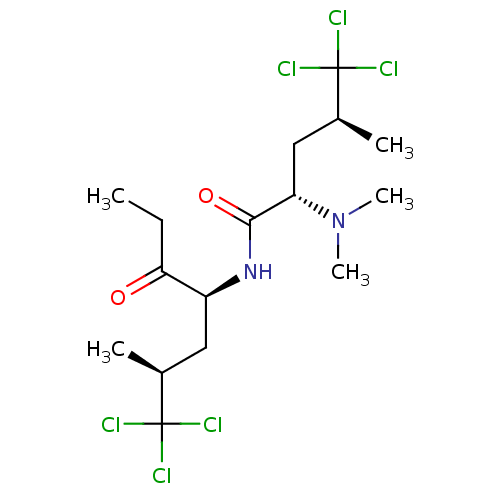
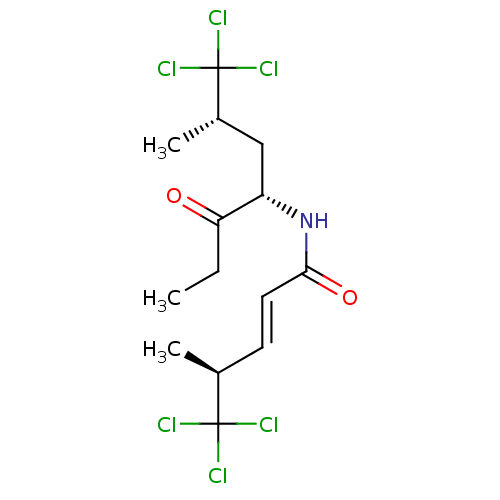
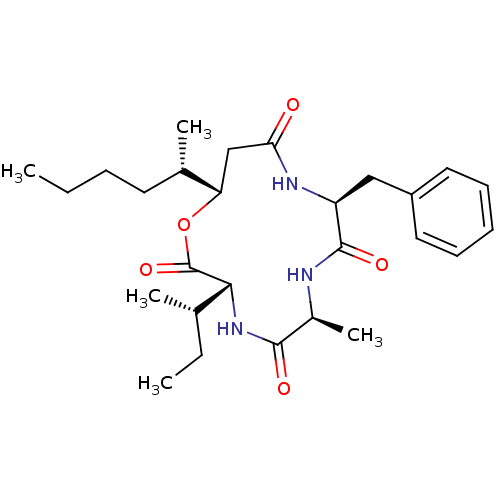
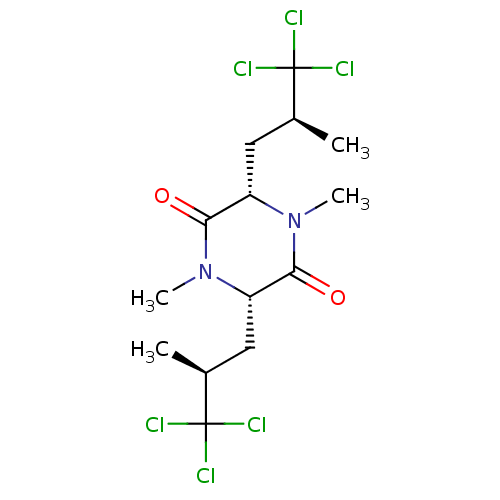
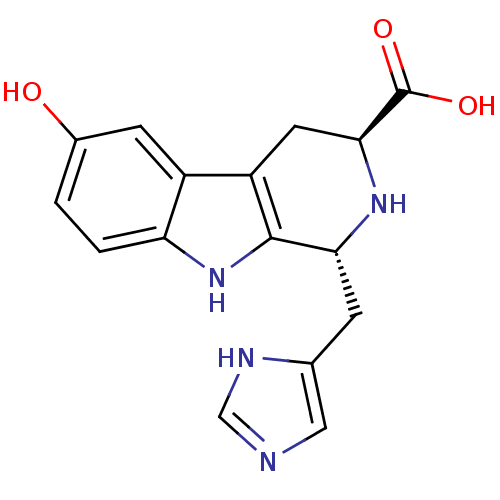
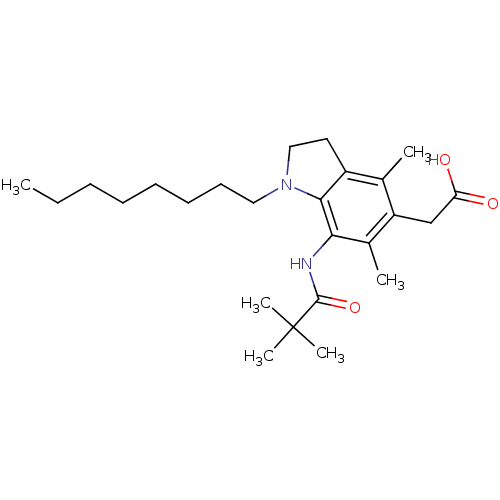
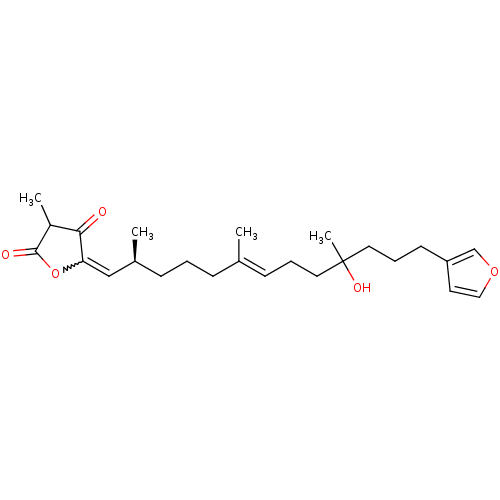
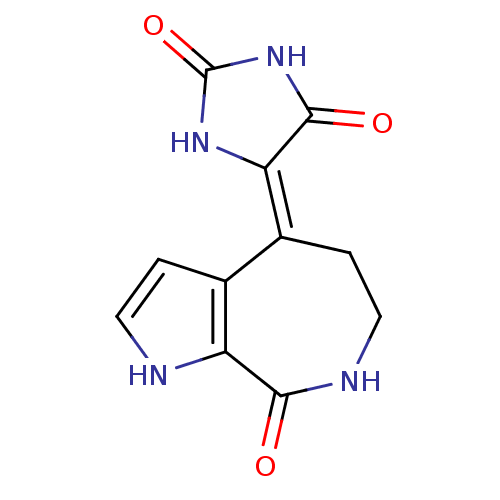
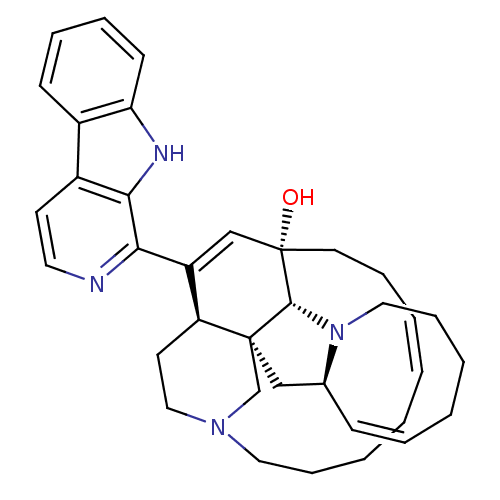
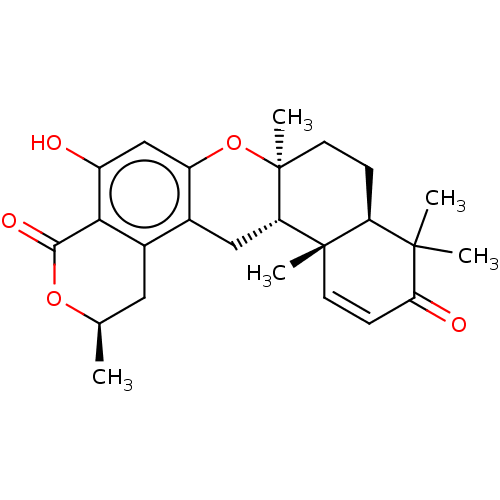
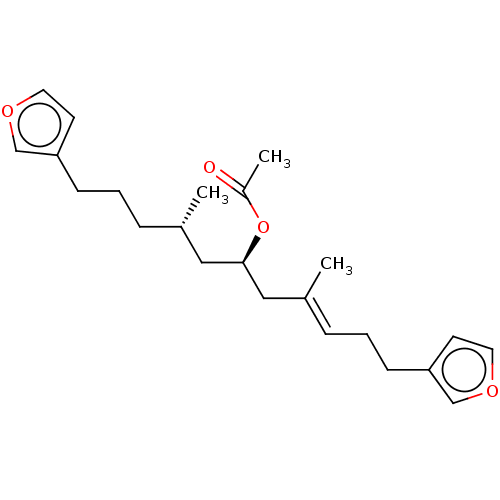
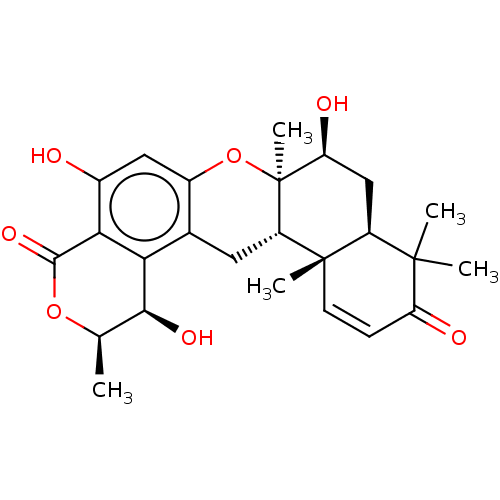
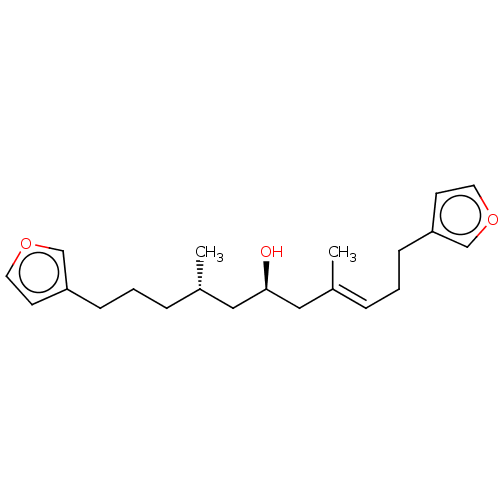
Affinity DataIC50: 32nMAssay Description:Inhibition of macrophage ACAT (unknown origin)More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibition of USP7 (unknown origin) using ubiquitin-EKL as substrateMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 780nMAssay Description:Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 860nMAssay Description:Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetUbiquitin-like modifier-activating enzyme 1(Homo sapiens (Human))
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of FLAG-tagged ubiquitin-activating enzyme E1 assessed as inhibition of GST-ubiquitin/FLAG-E1 intermediate formation by Western blot analy...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of African green monkey SOAT1 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of macrophage ACAT2 (unknown origin)More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min...More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 7(Homo sapiens (Human))
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of USP7 (unknown origin) using ubiquitin-EKL as substrateMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Non-competitive inhibition of human microsomal ACAT2 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of ACAT1 (unknown origin)More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of African green monkey SOAT1 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of African green monkey SOAT2 isolated from CHO cell microsomes assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 5 min...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Non-competitive inhibition of human microsomal ACAT1 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40E+3nMAssay Description:Non-competitive inhibition of microsomal ACAT in human MDM using [14C]oleoyl-CoA as substrate assessed as formation of cholesteryl [14C]-oleate after...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of human CD45 cytoplasmic domain (584 to 1281 residues) expressed in yeast using pNPP as substrate preincubated for 10 mins followed by su...More data for this Ligand-Target Pair
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of ACAT2 in human MDM assessed as inhibition of acetylated LDL-induced cholesterol ester accumulation after 1 hr by HPLC analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 9.90E+3nMAssay Description:Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human VHR expressed in Escherichia coli using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.46E+4nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate preincubated for 10 mins followed by substrate addition measured after 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 1.49E+4nMAssay Description:Inhibition of PTP1B (unknown origin)More data for this Ligand-Target Pair
TargetUbiquitin carboxyl-terminal hydrolase 21(Homo sapiens (Human))
Kumamoto University
Curated by ChEMBL
Kumamoto University
Curated by ChEMBL
Affinity DataIC50: 1.66E+4nMAssay Description:Inhibition of core catalytic domain of USP21 (unknown origin) using ubiquitin-Rh110 as substrateMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 2(Chlorocebus aethiops)
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of African green monkey SOAT2 expressed in CHO cells assessed as decrease in [14C]CE synthesis from [14C]oleic acid after 6 hrsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Tohoku Medical and Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.15E+4nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged human TCPTP expressed in Escherichia coli using pNPP as substrate preincubated for 10 min...More data for this Ligand-Target Pair