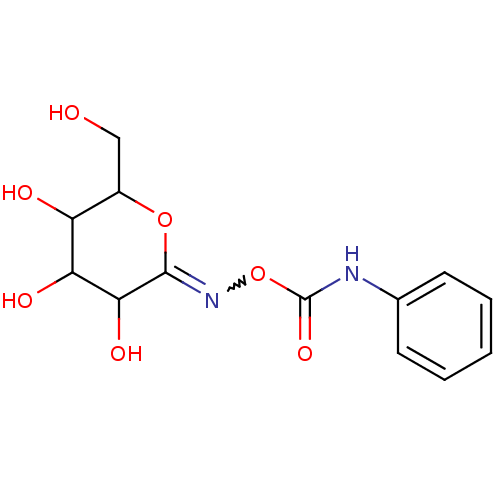
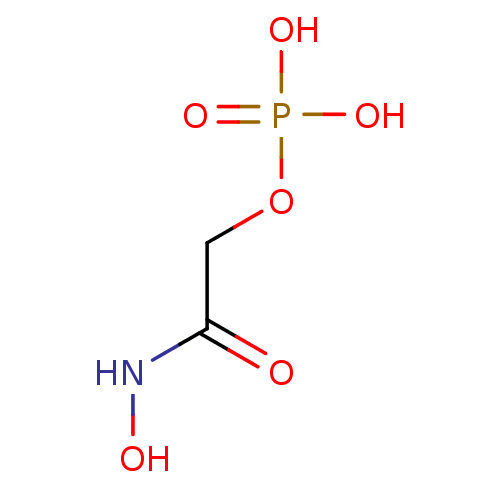
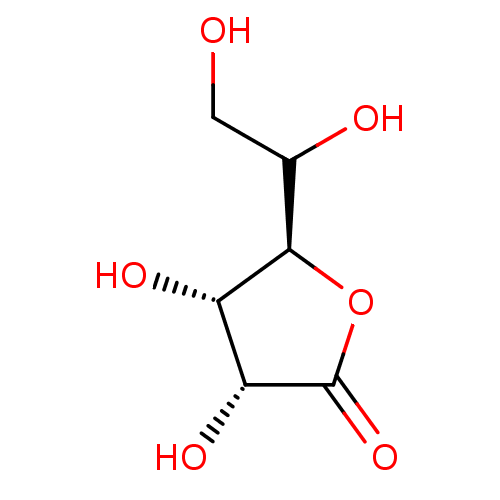
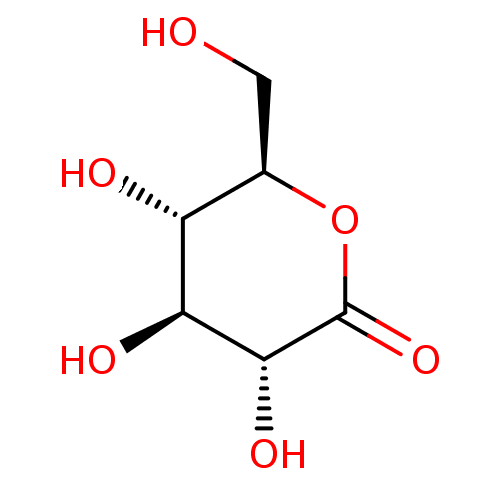
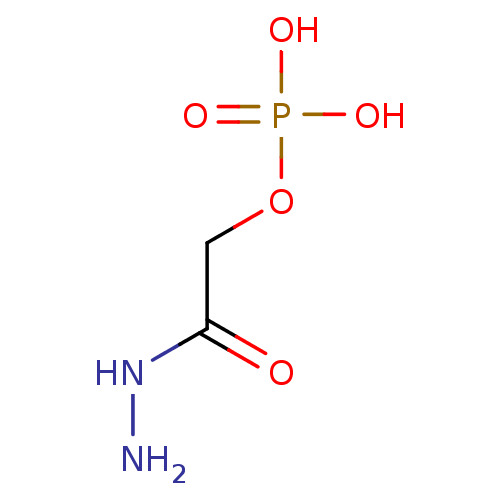
TargetBeta-mannosidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:The compound was tested for its inhibitory activity against beta-mannosidaseMore data for this Ligand-Target Pair
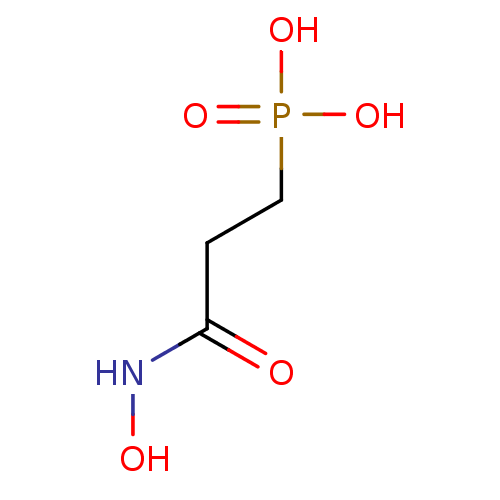
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:The compound was tested for its inhibitory activity against beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair
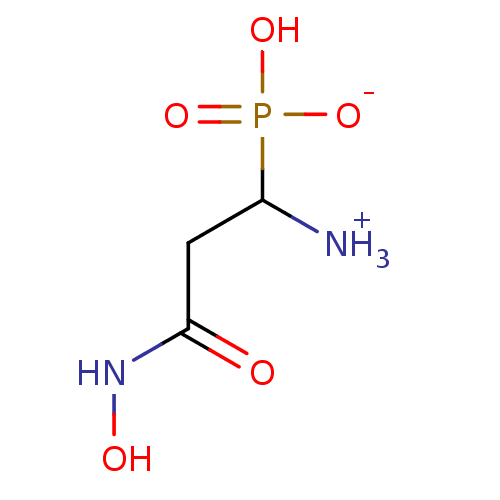
TargetBeta-mannosidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:The compound was tested for its inhibitory activity against beta-mannosidaseMore data for this Ligand-Target Pair
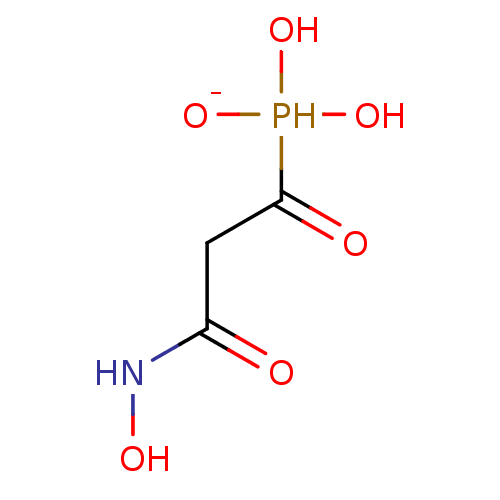
TargetBeta-mannosidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:The compound was tested for its inhibitory activity against beta-mannosidaseMore data for this Ligand-Target Pair
TargetLysosomal acid glucosylceramidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:The compound was tested for its inhibitory activity against beta-glucosidaseMore data for this Ligand-Target Pair
TargetLysosomal alpha-glucosidase(Homo sapiens (Human))
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
S.E.S.N.A.B., Pole Sciences et Technologie, Universit� de La Rochelle. therisod@icmo.u-psud.fr
Curated by ChEMBL
Affinity DataKi: 7.50E+4nMAssay Description:The compound was tested for its inhibitory activity against Alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.11E+5nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+5nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of triosephosphate isomeraseMore data for this Ligand-Target Pair