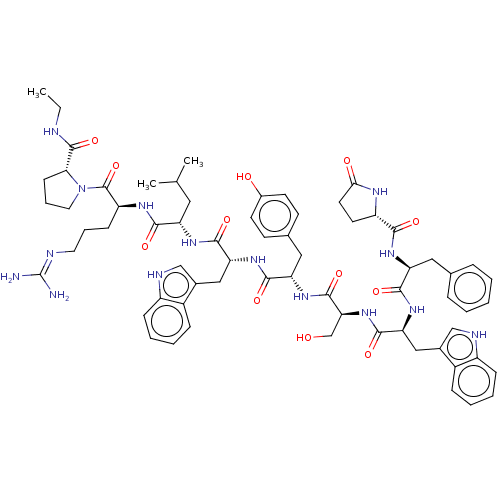
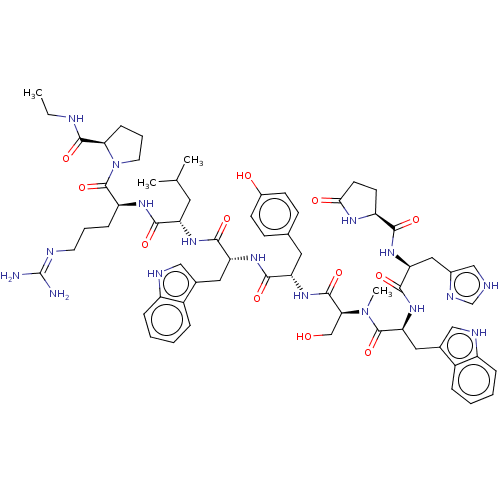
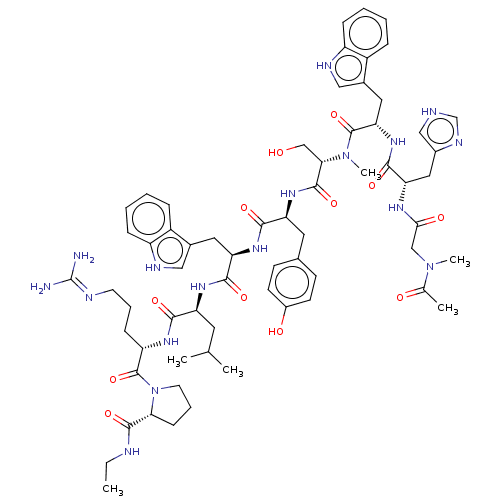
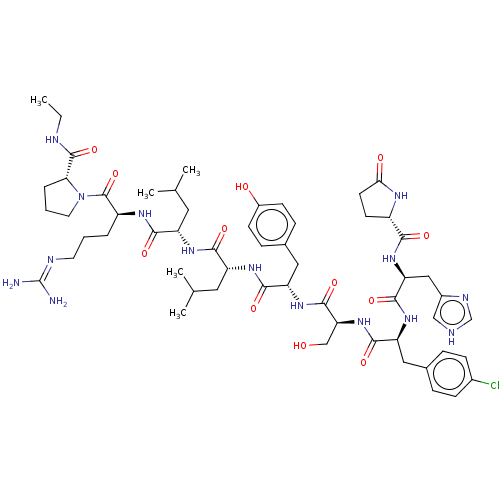
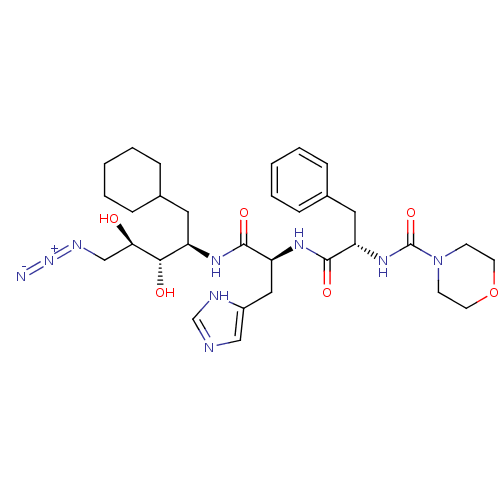
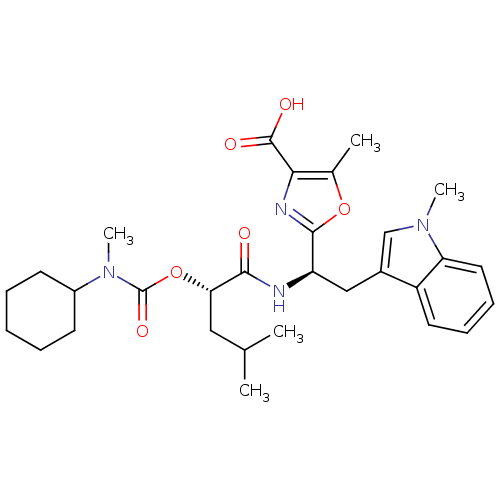
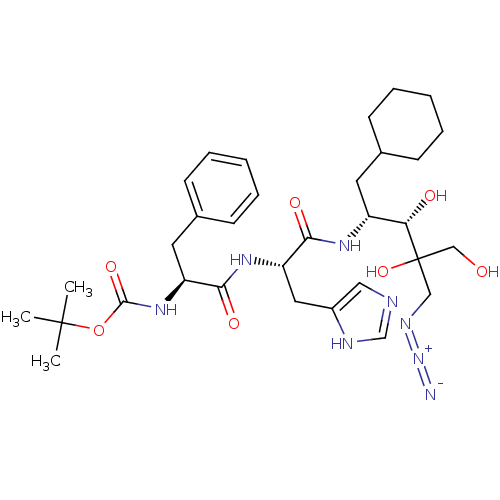
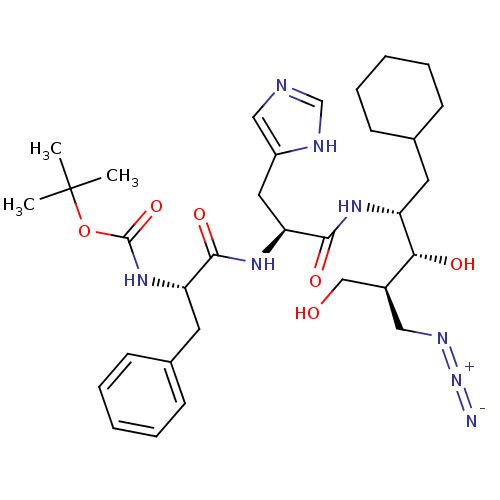
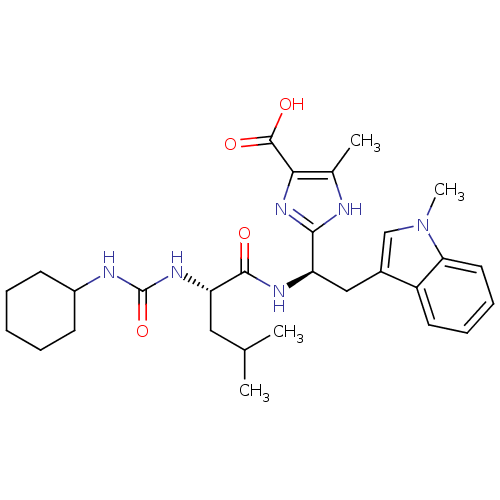
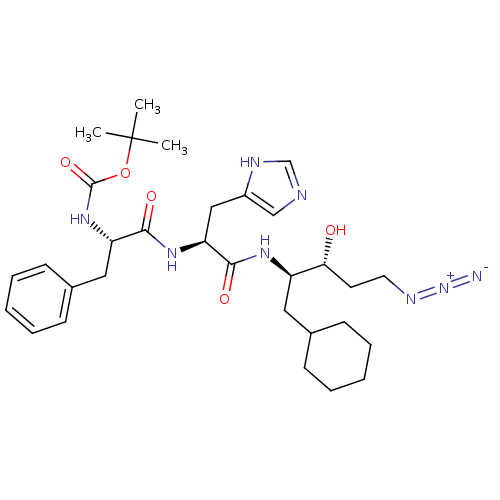
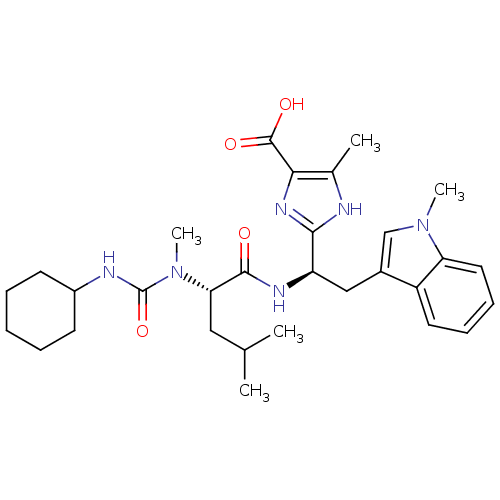
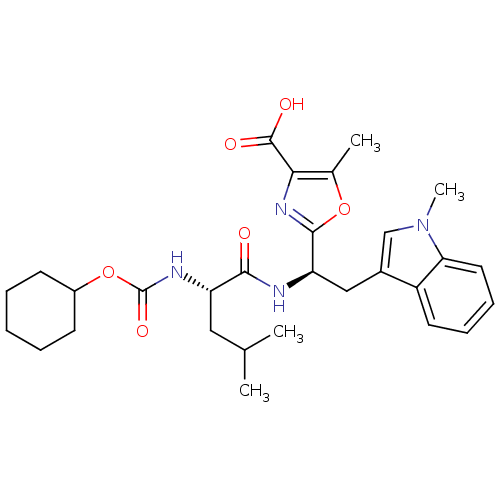
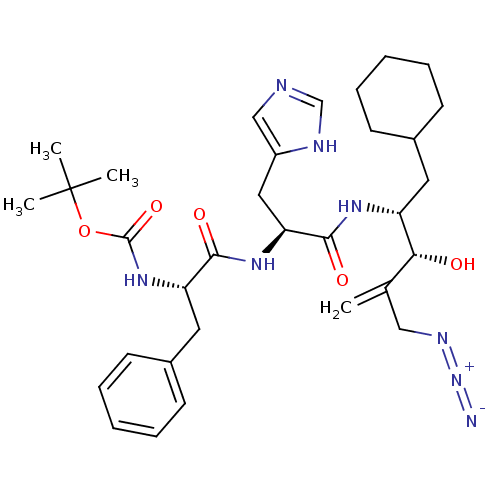
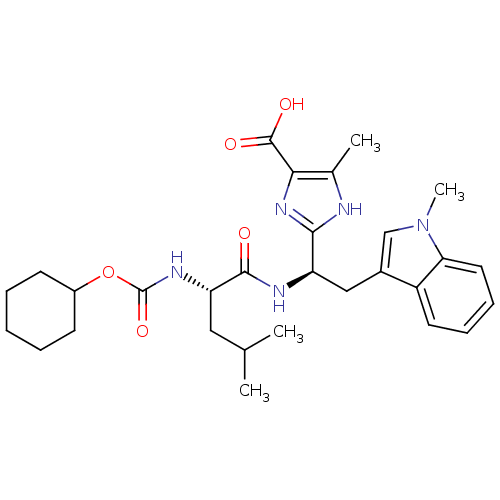
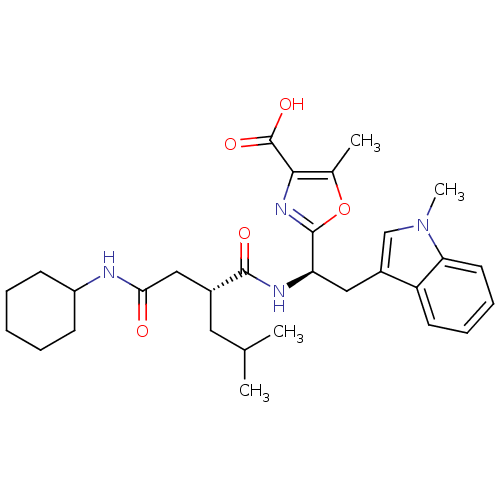
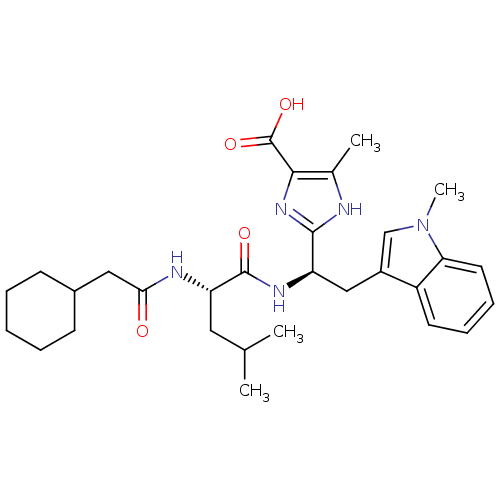
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0245nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0427nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0776nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0933nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.155nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.186nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.209nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
TargetLutropin-choriogonadotropic hormone receptor(Rattus norvegicus)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataKi: 9.30nMAssay Description:Negative logarithm of equilibrium dissociation constant in the rat pituitary luteinizing releasing hormone receptor binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMpH: 6.0Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMpH: 6.0Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMpH: 6.0Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMpH: 6.0Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 6.0Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 7.4Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 7.4Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 6.0Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 7.60nMpH: 7.4Assay Description:Inhibitory activity against purified human plasma renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.4Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.4Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 7.4Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 20nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 370nMpH: 6.0Assay Description:Inhibitory activity against purified human renal renin at pH 6.0More data for this Ligand-Target Pair
Affinity DataIC50: 450nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Inhibitory activity was evaluated against human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMpH: 6.0Assay Description:Inhibitory activity against human plasma renin at pH 7.4More data for this Ligand-Target Pair