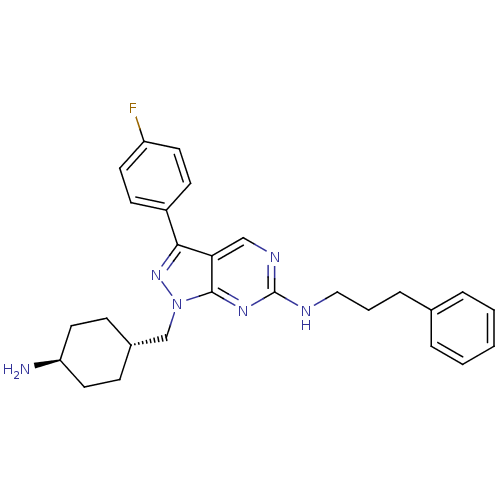
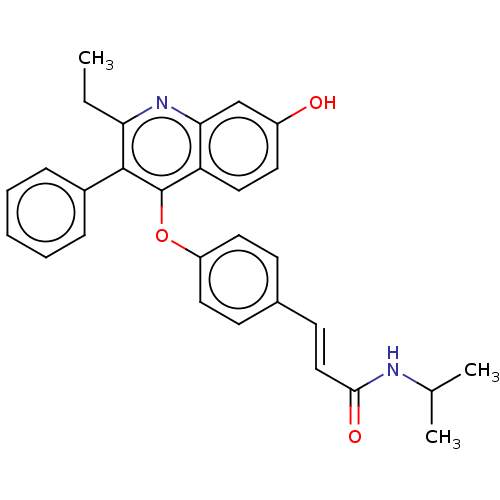
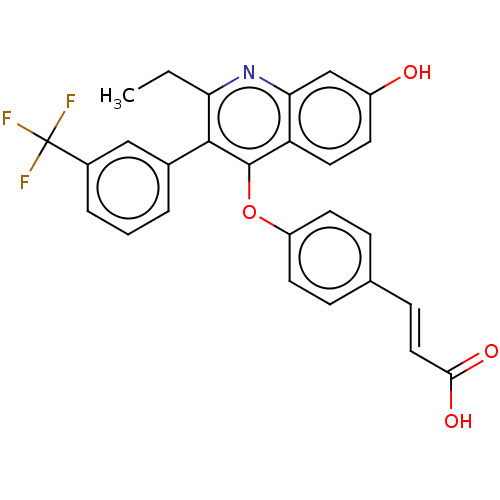
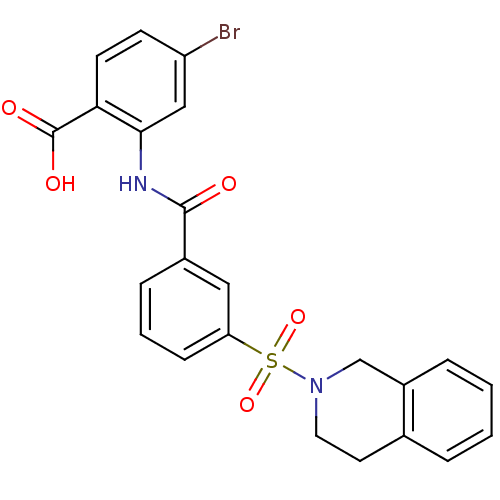
Affinity DataKi: 0.560nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
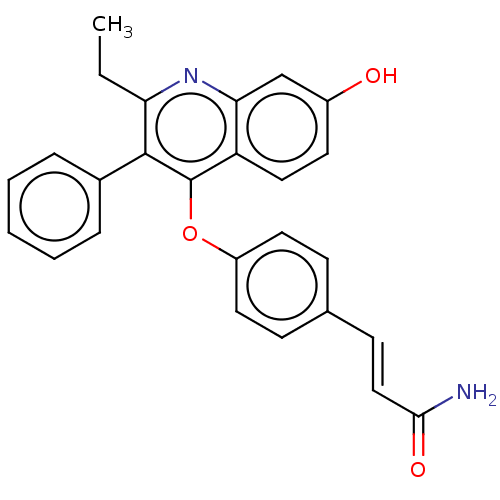
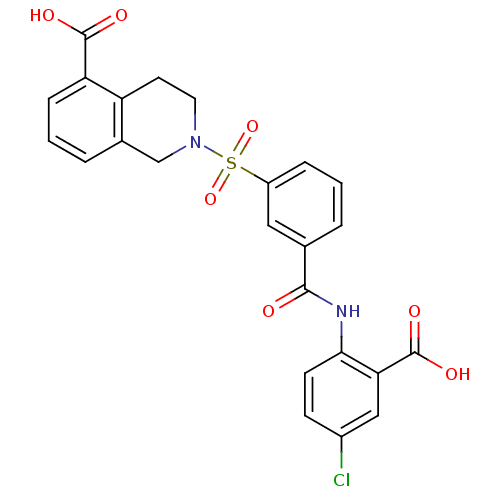
Affinity DataKi: 0.730nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
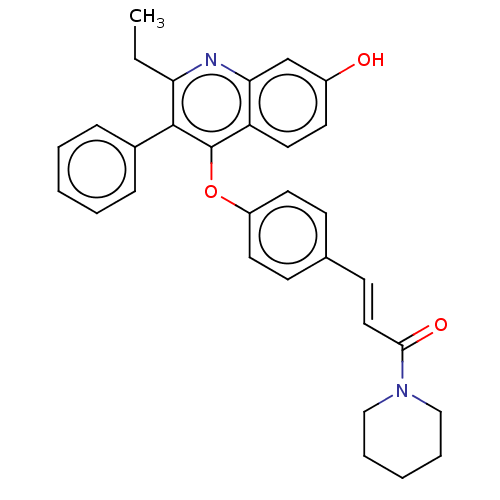
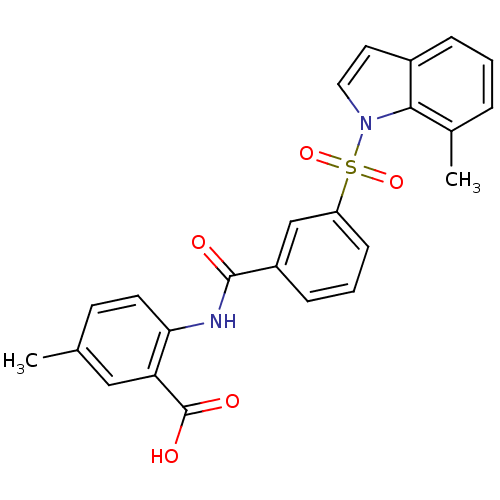
Affinity DataKi: 1.40nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
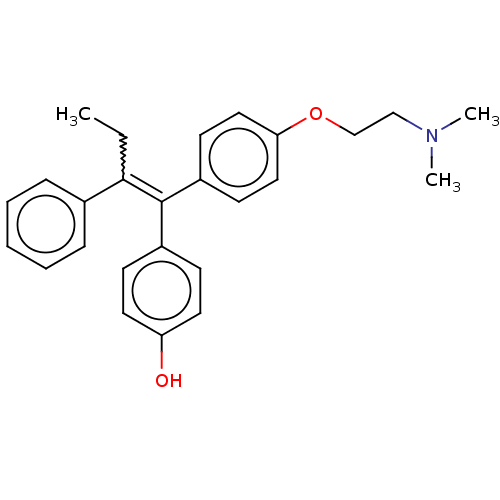
Affinity DataKi: 2nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
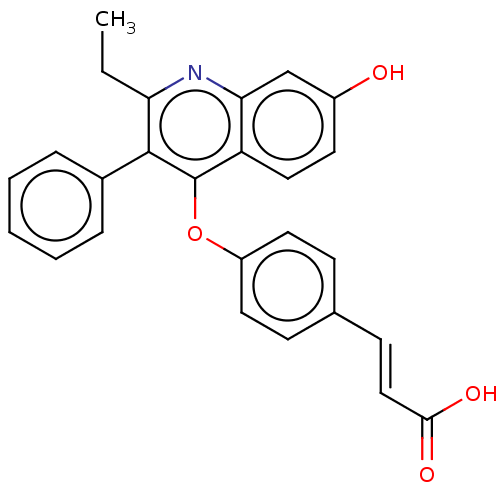
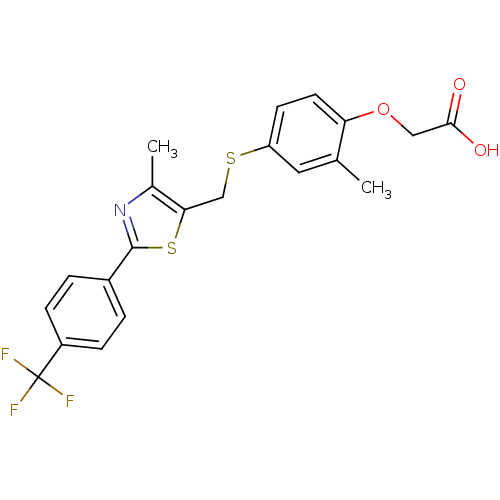
Affinity DataKi: 2nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
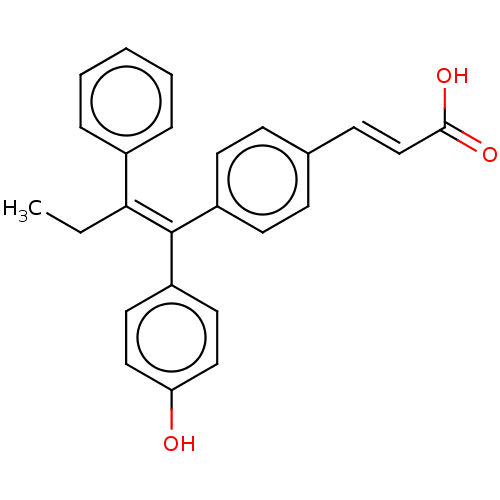
Affinity DataKi: 3.20nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equationMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 7.90nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 16nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 126nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 251nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 631nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 1.59E+3nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 1.59E+3nMAssay Description:Binding affinity for human estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataKi: 3.16E+3nMAssay Description:Binding affinity for human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:pIC50 for 1 nM estradiol-induced Ishikawa cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assayMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:pIC50 for 1 nM estradiol-induced Ishikawa cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Inhibition of Tyro3 using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:pIC50 for 1 nM estradiol-induced Ishikawa cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assayMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:pIC50 for 1 nM estradiol-induced Ishikawa cell proliferationMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
GlaxoSmithKline Research & Development
Curated by ChEMBL
GlaxoSmithKline Research & Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:pIC50 for 1 nM estradiol-induced Ishikawa cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nM EC50: 1.26E+3nMpH: 7.0 T: 2°CAssay Description:Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga...More data for this Ligand-Target Pair
Affinity DataIC50: 4nM EC50: 79nMAssay Description:Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga...More data for this Ligand-Target Pair
Affinity DataIC50: 5nM EC50: 3nMpH: 7.0 T: 2°CAssay Description:Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga...More data for this Ligand-Target Pair
Affinity DataIC50: 5nM EC50: 158nMpH: 7.0 T: 2°CAssay Description:Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga...More data for this Ligand-Target Pair
Affinity DataIC50: 5nM EC50: 158nMpH: 7.0 T: 2°CAssay Description:Competition-binding curves for test compounds were determined with expressed human PPAR LBD. Plots of inhibitor concentration versus cpm of radioliga...More data for this Ligand-Target Pair

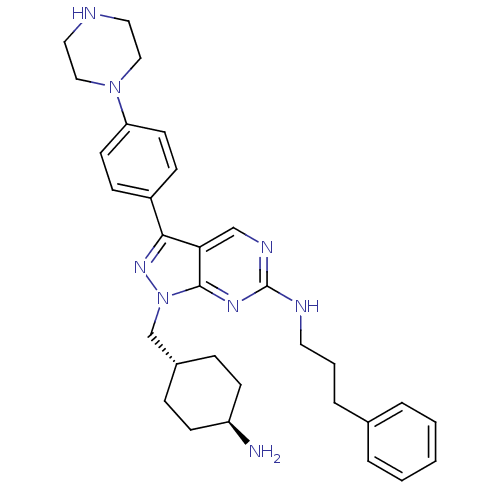
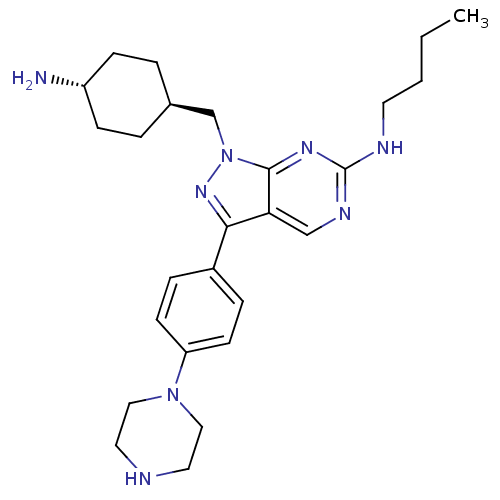
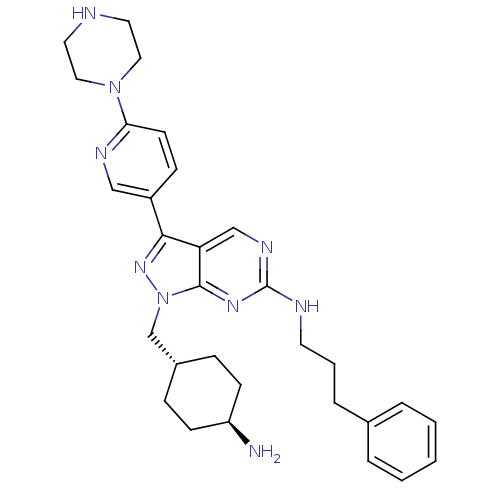
 3D Structure (crystal)
3D Structure (crystal)