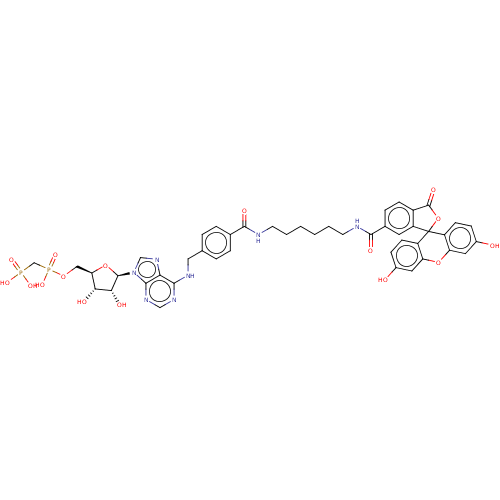
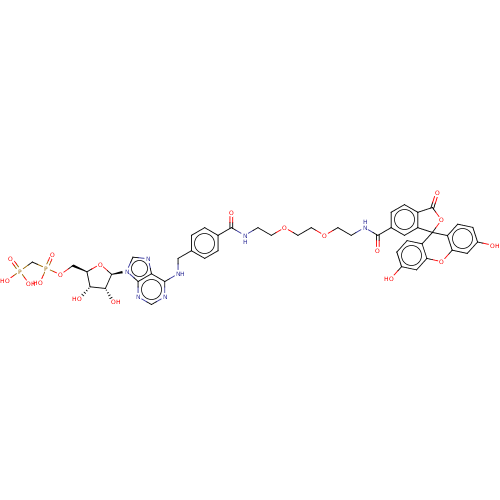
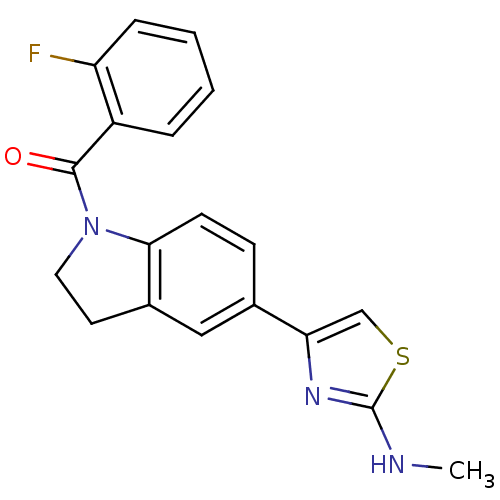
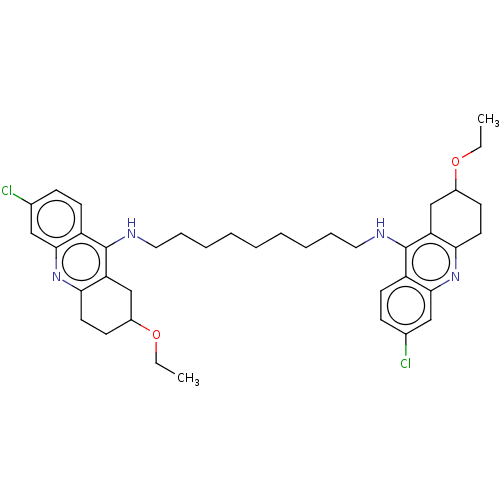
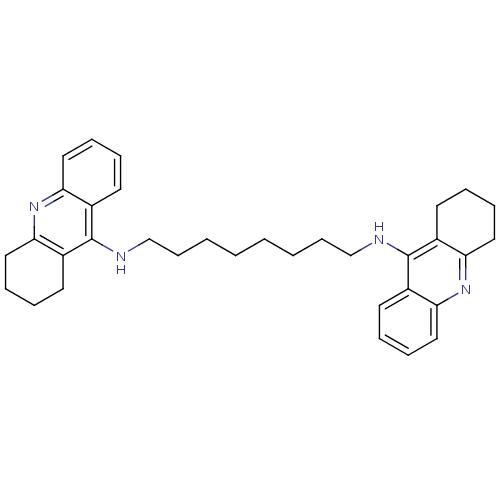
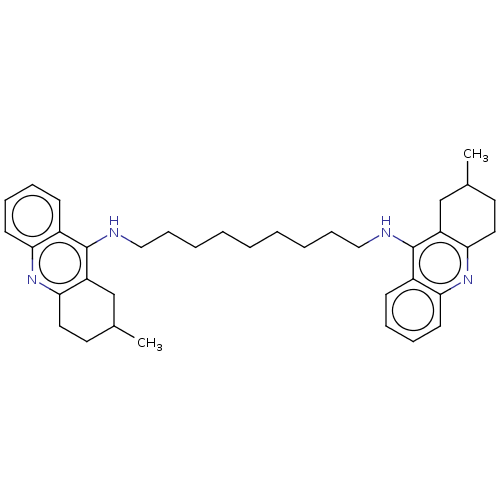
Affinity DataKi: 0.381nMAssay Description:Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ...More data for this Ligand-Target Pair
Affinity DataKi: 0.746nMAssay Description:Inhibition of recombinant rat CD73 expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ...More data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Inhibition of CD73 in human MDA-MB-231 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for ...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of recombinant rat CD73 expressed in Sf9 cells using [2,8-3H]-AMP as substrate incubated for 25 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetTrypanothione reductase(Trypanosoma cruzi)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
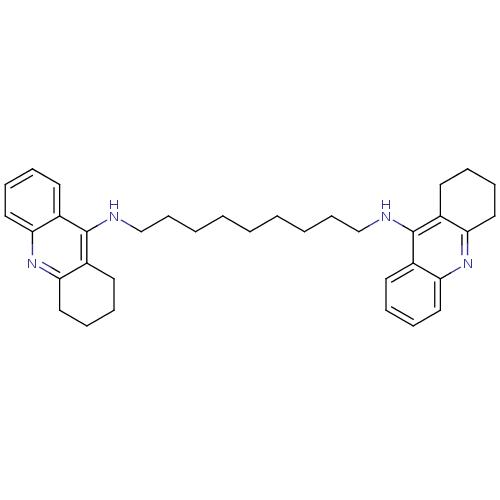
Affinity DataKi: 400nMAssay Description:Competitive inhibition of Trypanosoma cruzi trypanothione reductase using varying levels of trypanothione disulfide as substrate by Lineweaver--burk ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK2 [166-489](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
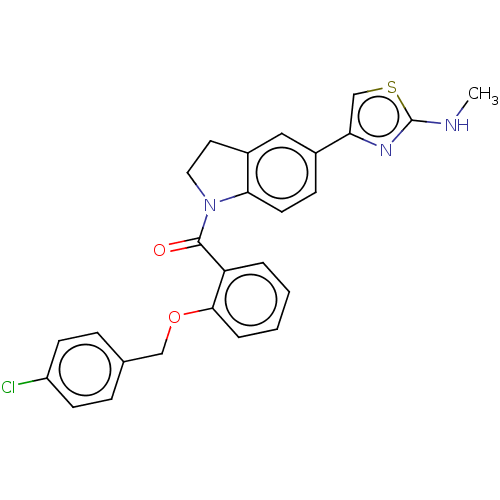
Affinity DataIC50: 50nMpH: 7.3Assay Description:The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [1-491](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 64nMpH: 7.3Assay Description:The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [1-491](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 150nMpH: 7.5Assay Description:A mixture of fluorescein labeled OSR1 peptide substrate (Toray Research Center, Inc.) and ATP was prepared with final concentrations of 10 and 25 ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [1-491](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 180nMpH: 7.5Assay Description:A mixture of fluorescein labeled OSR1 peptide substrate (Toray Research Center, Inc.) and ATP was prepared with final concentrations of 10 and 25 ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK4 [1-444](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 187nMpH: 7.3Assay Description:The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK3 [1-434](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 377nMpH: 7.3Assay Description:The assay utilized 5 to 10 nM of WNK1−4 protein compared to 25 nM used for mobility shift assay, enabling a more accurate comparison of selecti...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [198-401](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 5.70E+3nMAssay Description:For hit validation, eight compound replicates were made from the original stock dilution plates. The Cybi-WellTM dispenser was used to prepare 1.5 &#...More data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [198-401](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 8.80E+3nMAssay Description:For hit validation, eight compound replicates were made from the original stock dilution plates. The Cybi-WellTM dispenser was used to prepare 1.5 &#...More data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 1.33E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [198-401](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 1.60E+4nMAssay Description:For hit validation, eight compound replicates were made from the original stock dilution plates. The Cybi-WellTM dispenser was used to prepare 1.5 &#...More data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 1.69E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 1.81E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 1.94E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 2.66E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetCysteine protease(Trypanosoma brucei rhodesiense)
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany.
Curated by ChEMBL
Affinity DataIC50: 2.66E+4nMAssay Description:Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate measured for 10 mins by spectrofluorometric analysisMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase WNK1 [198-401](Homo sapiens (Human))
Novartis Institutes for BioMedical Research, Inc.
Novartis Institutes for BioMedical Research, Inc.
Affinity DataIC50: 8.00E+4nMAssay Description:For hit validation, eight compound replicates were made from the original stock dilution plates. The Cybi-WellTM dispenser was used to prepare 1.5 &#...More data for this Ligand-Target Pair

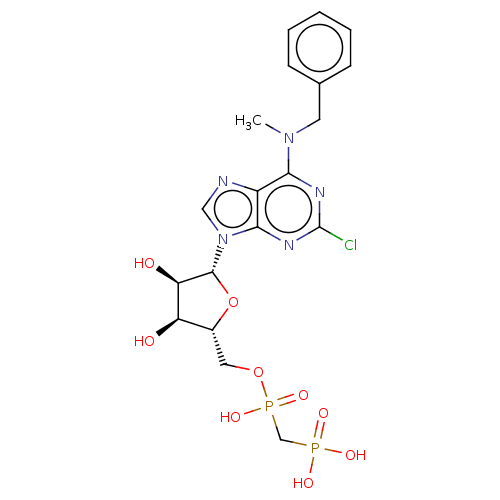
 3D Structure (crystal)
3D Structure (crystal)