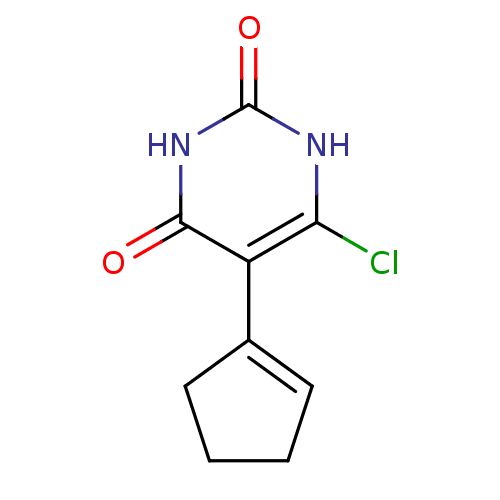
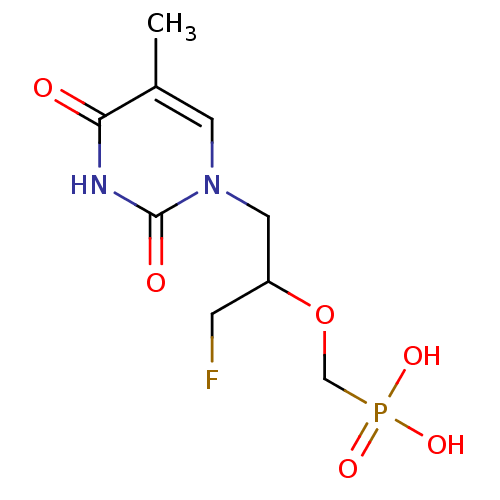
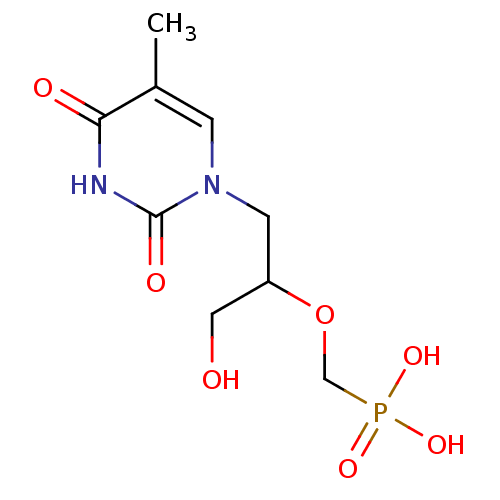
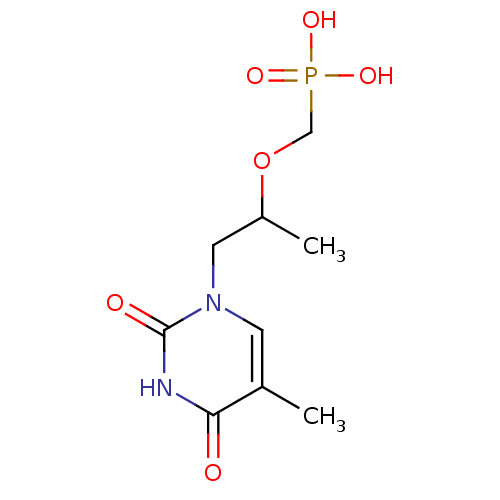
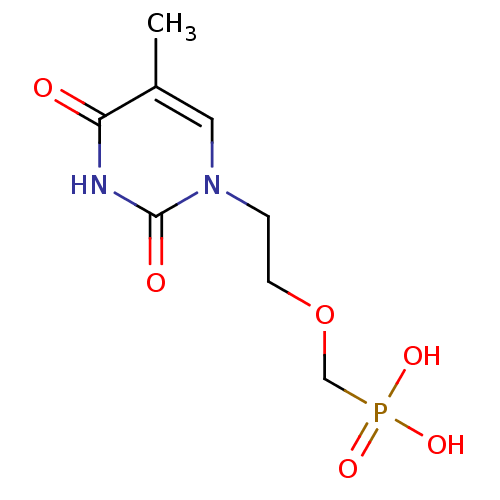
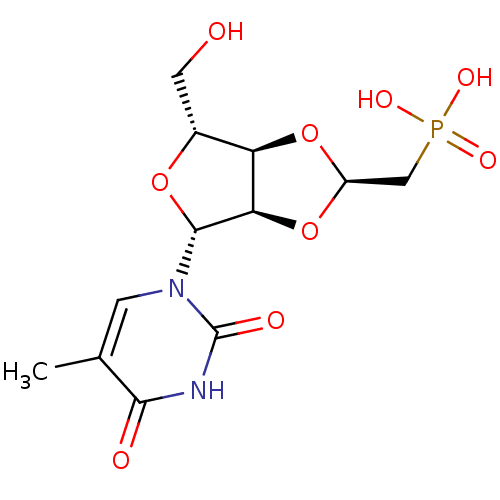
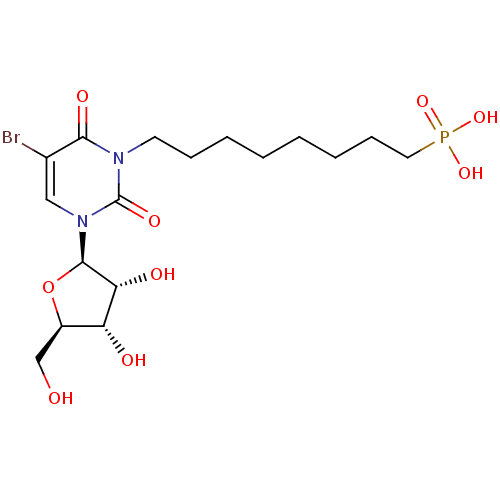
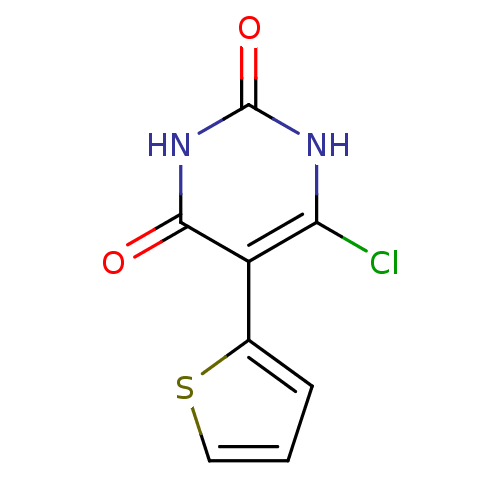
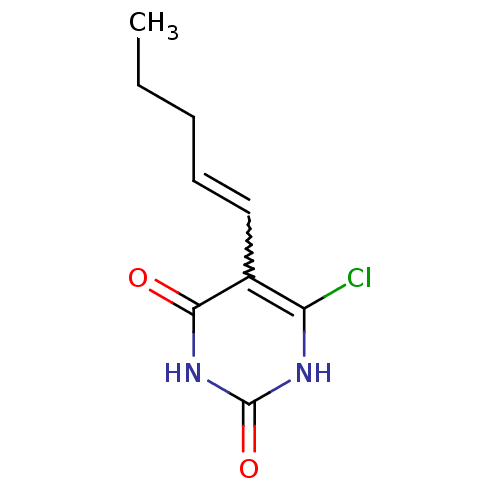
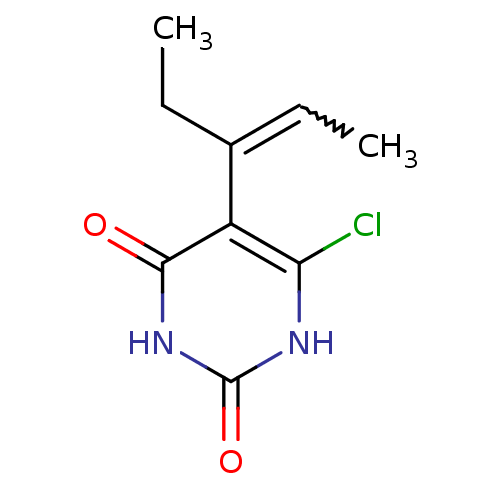
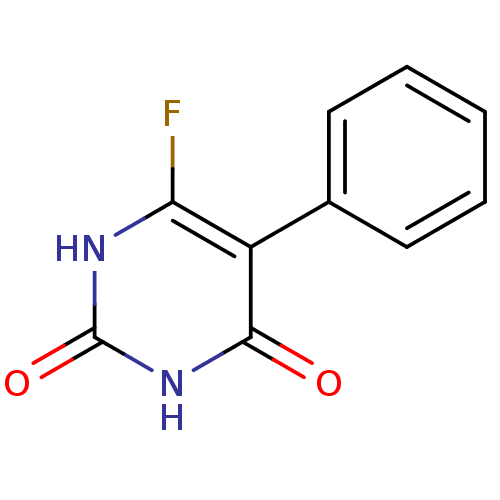
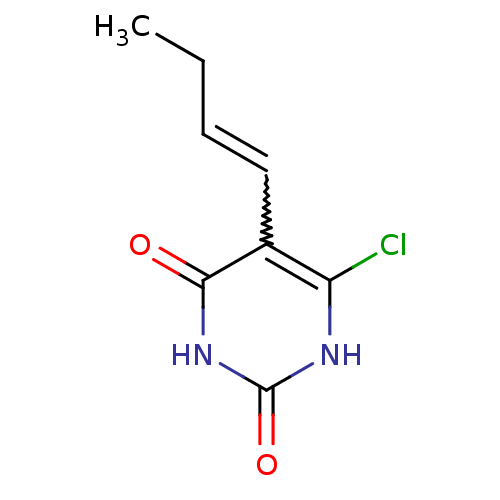
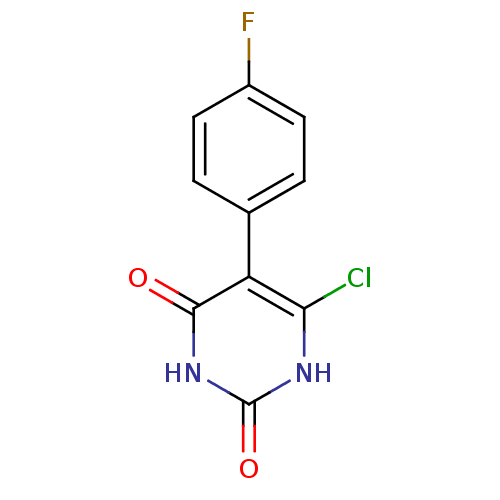
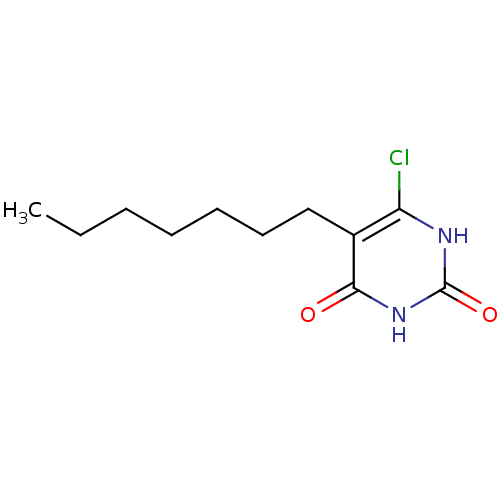
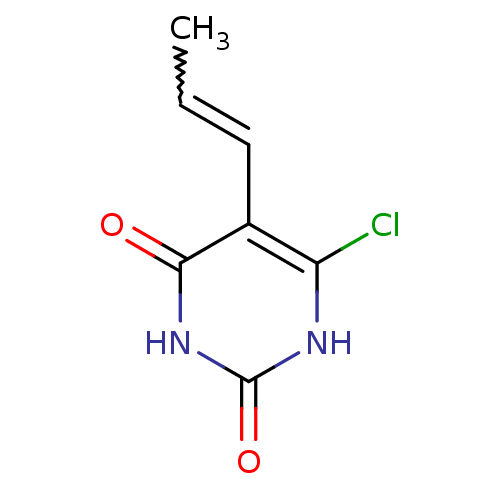
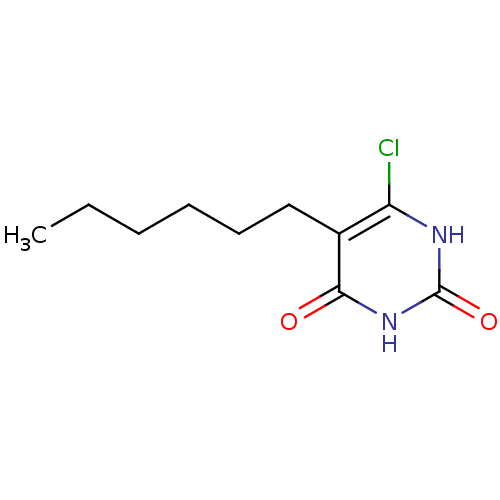
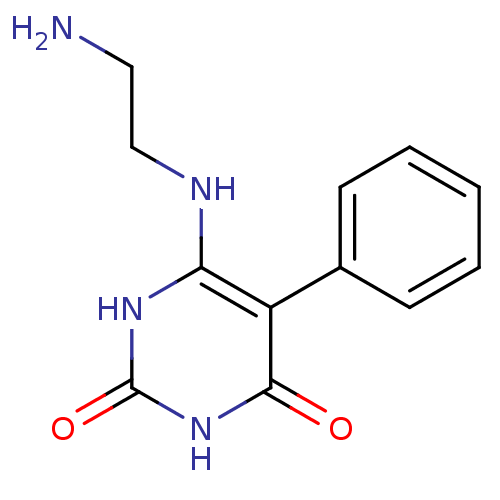
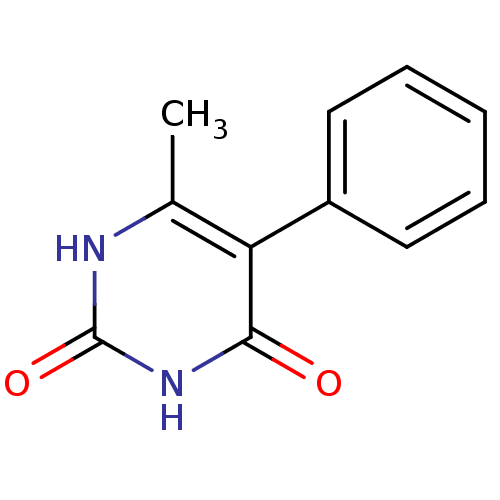
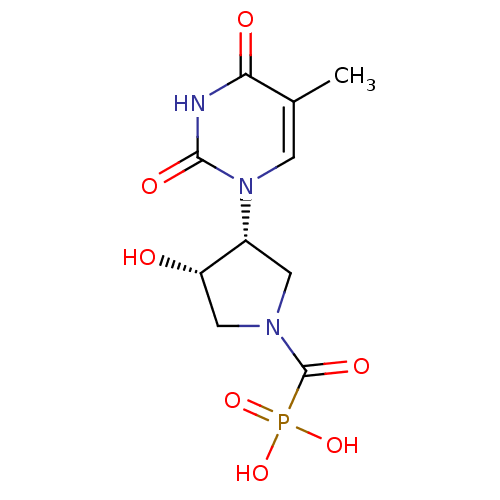
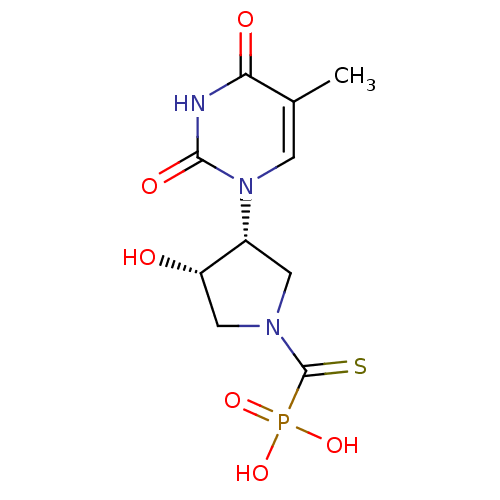
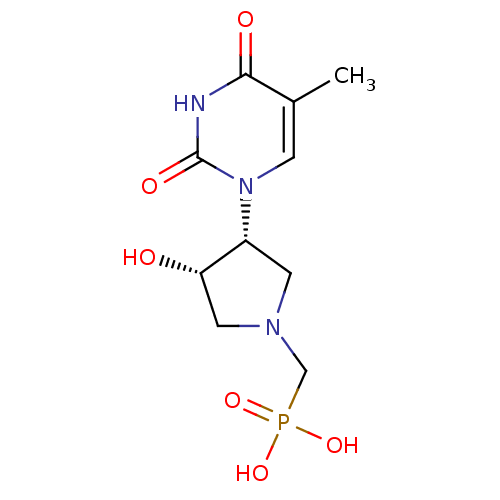
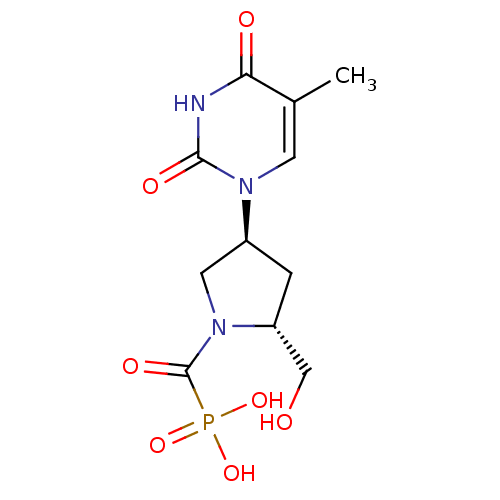
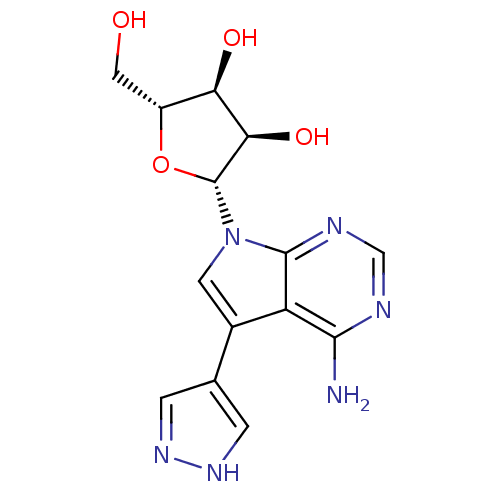
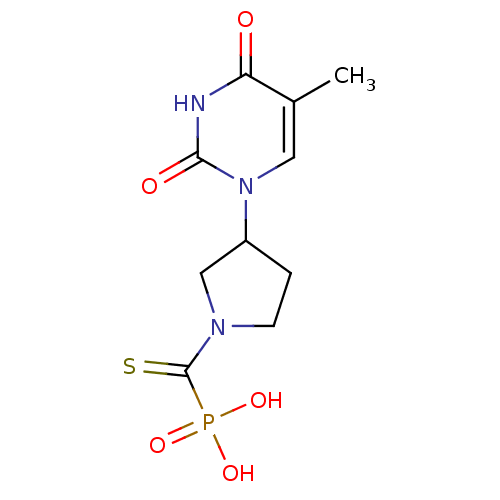
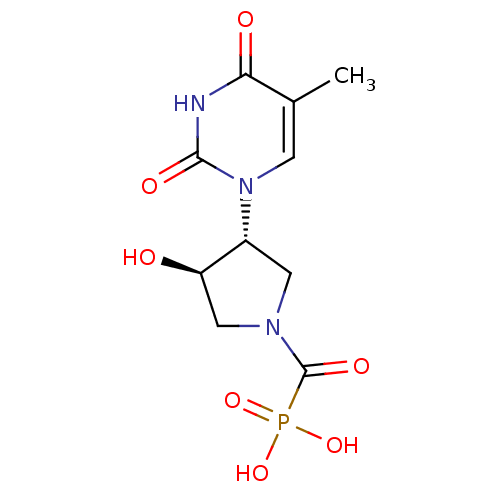
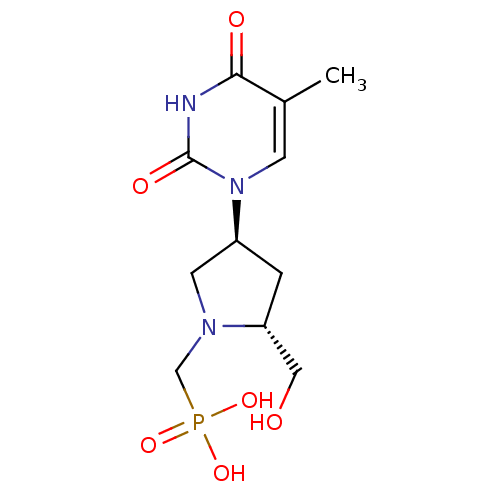
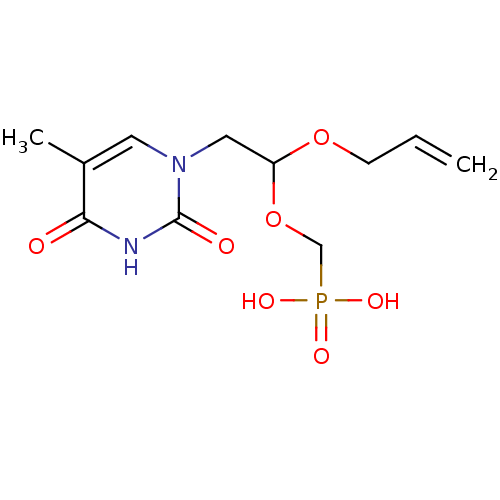
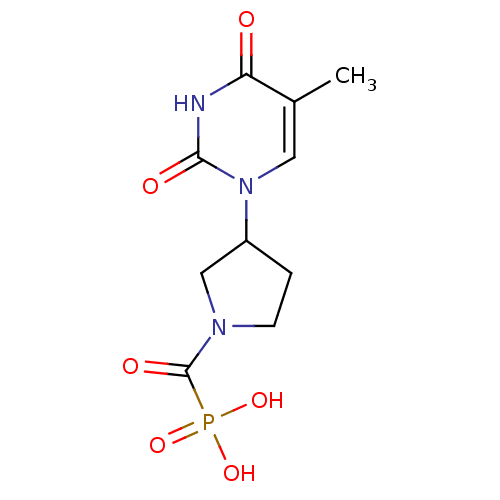
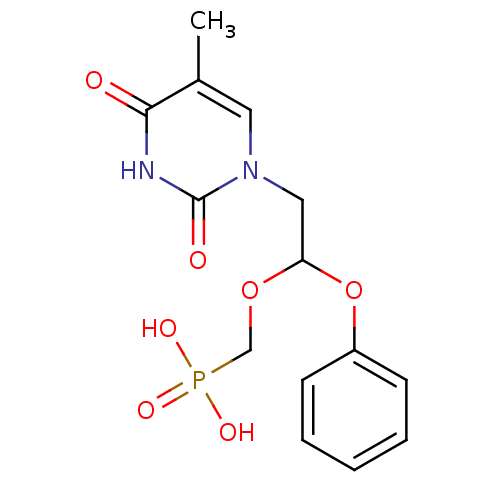
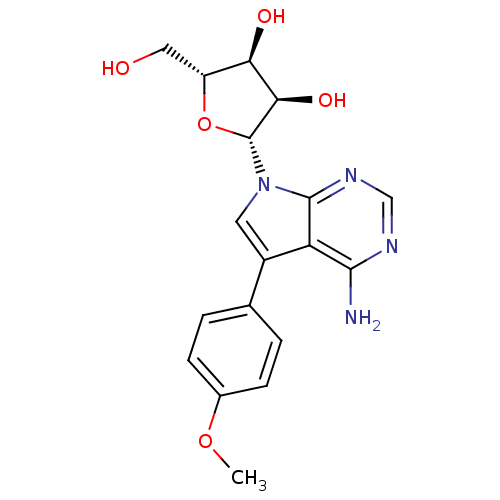
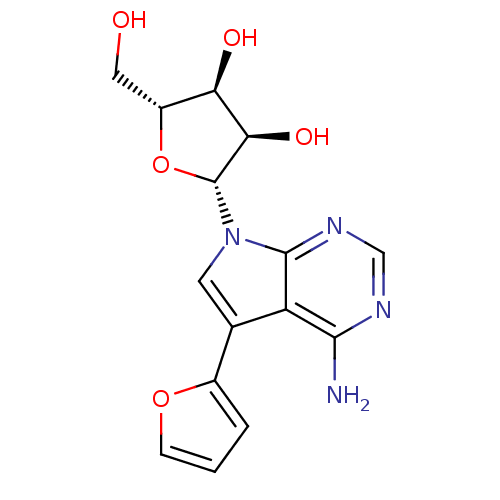
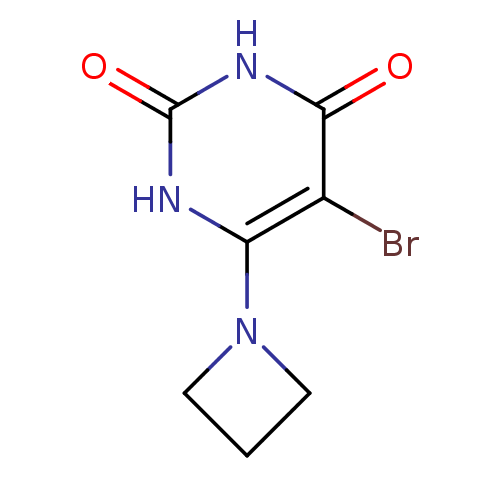
Affinity DataKi: 1.30nM ΔG°: -52.8kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 200nM ΔG°: -39.8kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 236nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataKi: 280nM ΔG°: -38.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 400nM ΔG°: -38.0kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 410nM ΔG°: -37.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 420nM ΔG°: -37.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 490nM ΔG°: -37.5kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 500nM ΔG°: -37.4kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 630nM ΔG°: -36.8kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 710nM ΔG°: -36.5kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.03E+3nM ΔG°: -35.5kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+3nM ΔG°: -35.5kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.17E+3nM ΔG°: -35.2kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.21E+3nM ΔG°: -35.1kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.74E+3nM ΔG°: -34.2kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 1.83E+3nM ΔG°: -34.1kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 3.01E+3nM ΔG°: -32.8kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 3.34E+3nM ΔG°: -32.5kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 3.67E+3nM ΔG°: -32.3kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 4.59E+3nM ΔG°: -31.7kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 4.65E+3nM ΔG°: -31.7kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: 5.81E+3nM ΔG°: -31.1kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-27.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-27.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-27.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-27.9kJ/molepH: 6.4 T: 2°CAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human ADK expressed in Escherichia coli BL21(DE3) cells using [3H]adenosine by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetThymidine phosphorylase(Rattus norvegicus)
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphateMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human ADK using [3H]-adenosine by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b...More data for this Ligand-Target Pair
TargetAdenosine kinase(Homo sapiens (Human))
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Academy of Sciences of the Czech Republic
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of human ADK expressed in Escherichia coli BL21(DE3) cells using [3H]adenosine by liquid scintillation counting methodMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)