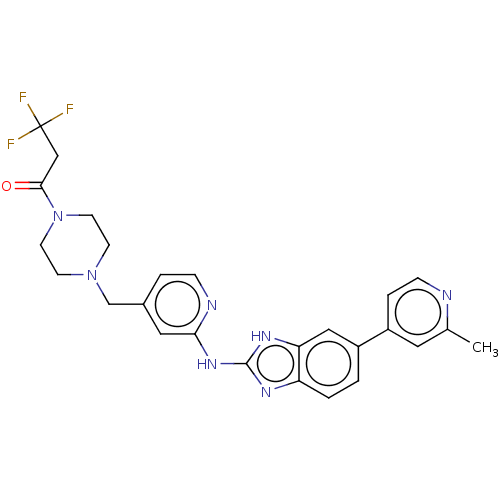
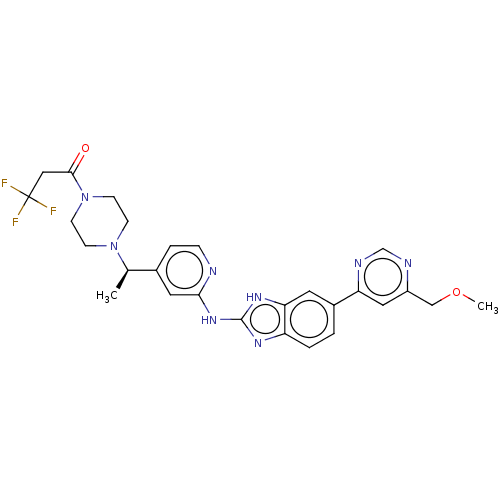
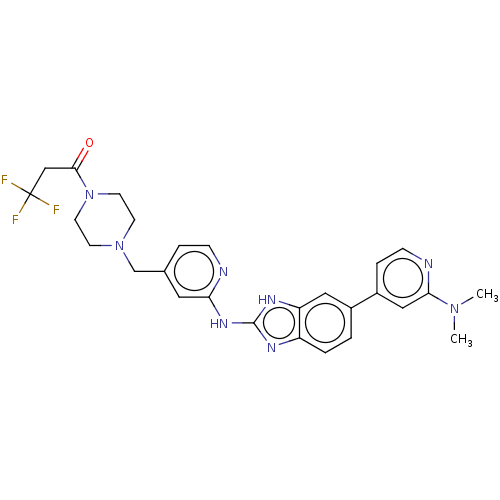
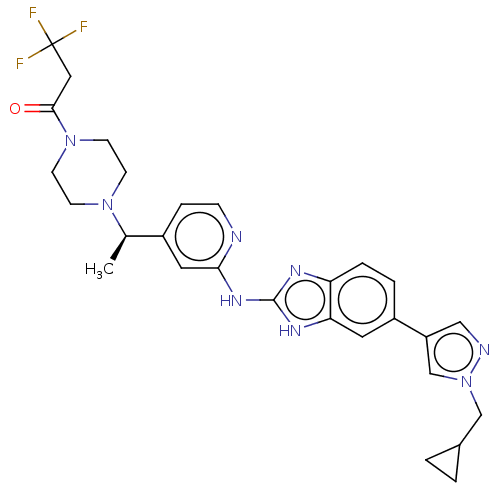
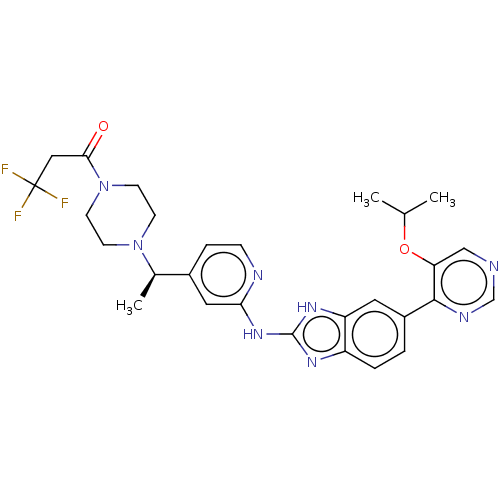
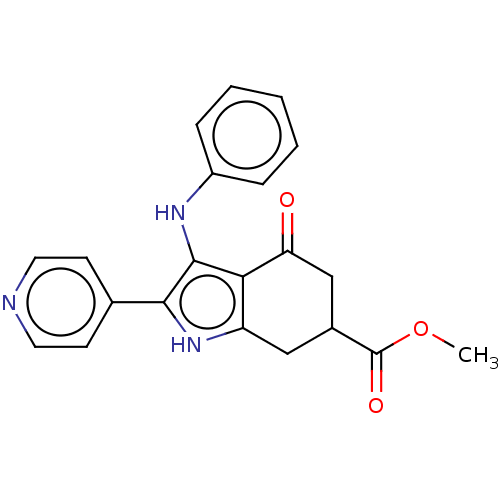
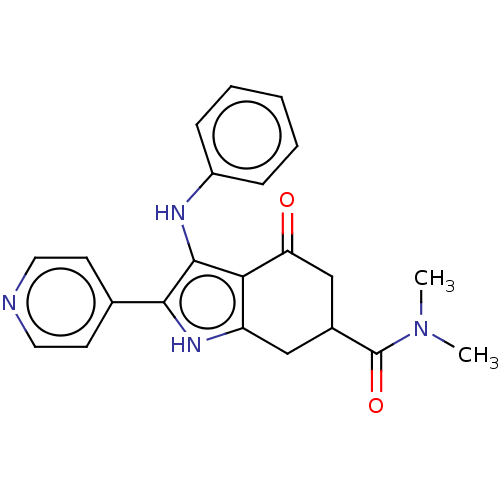
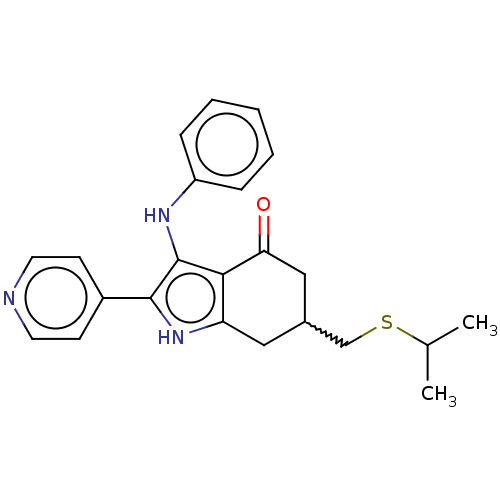
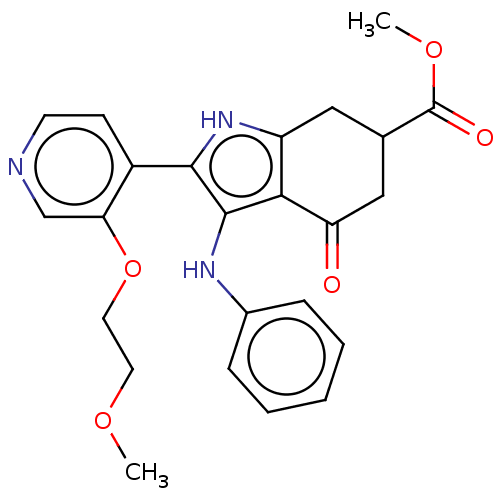
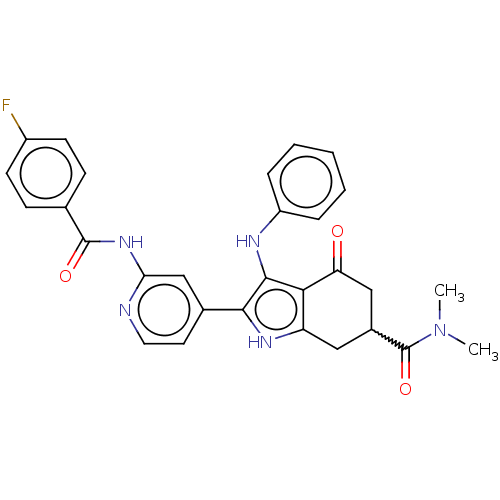
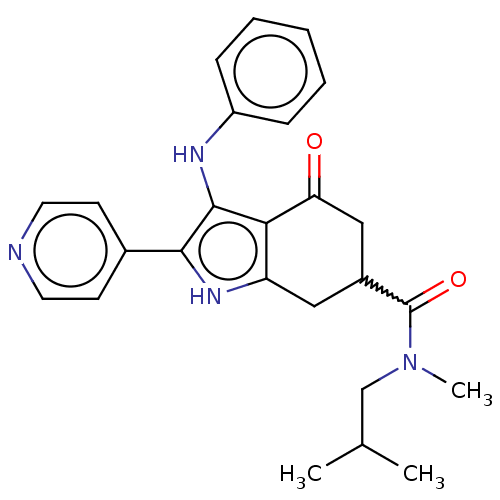
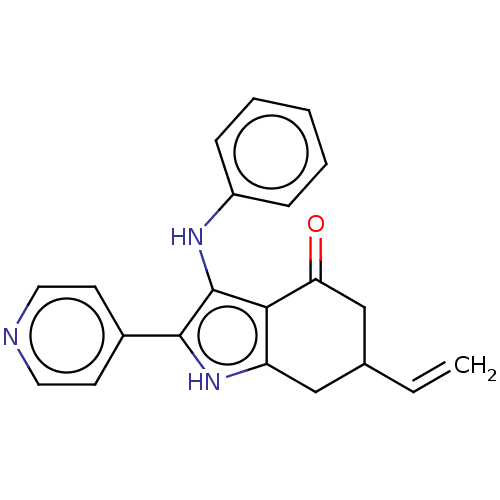
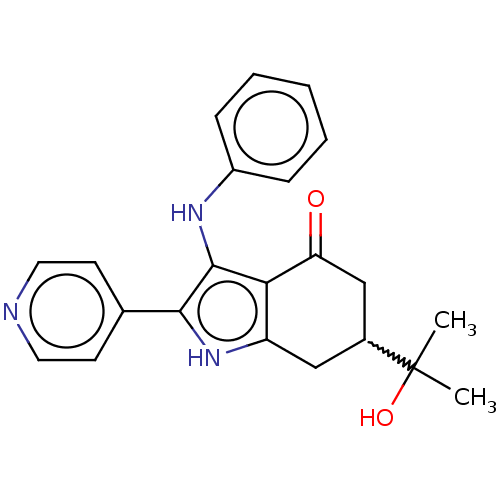
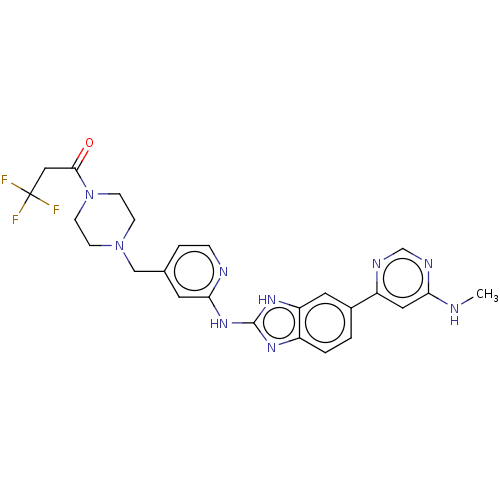
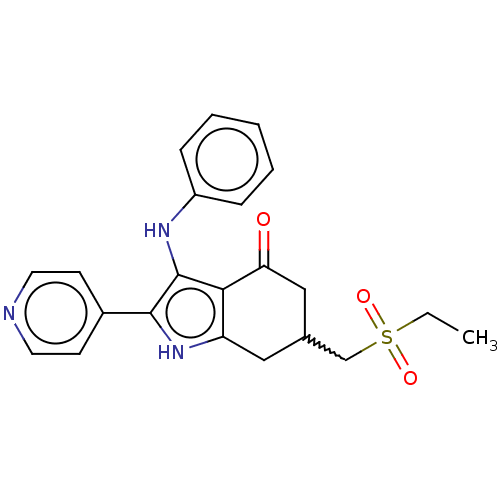
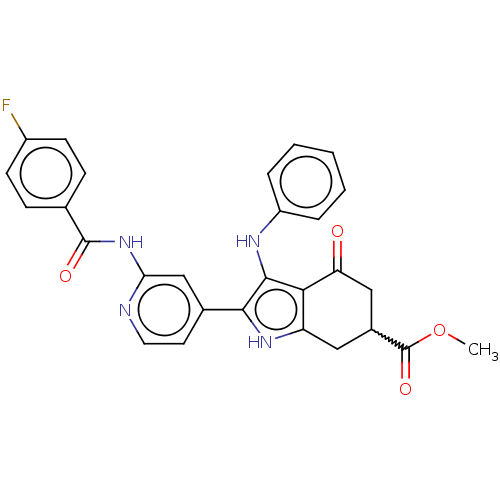
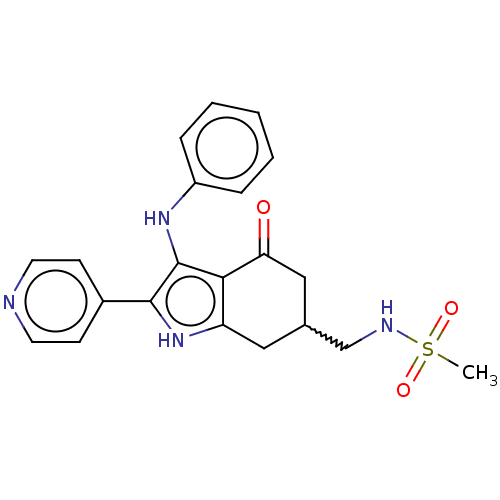
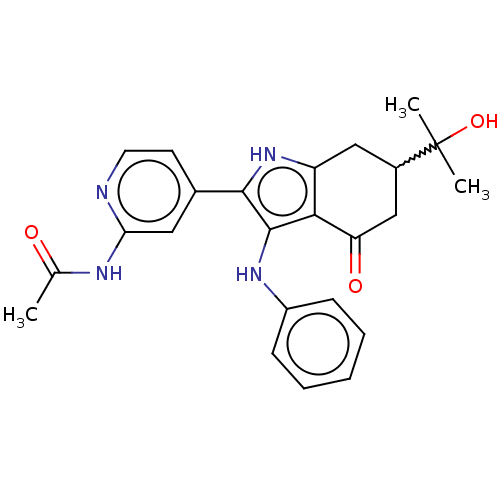
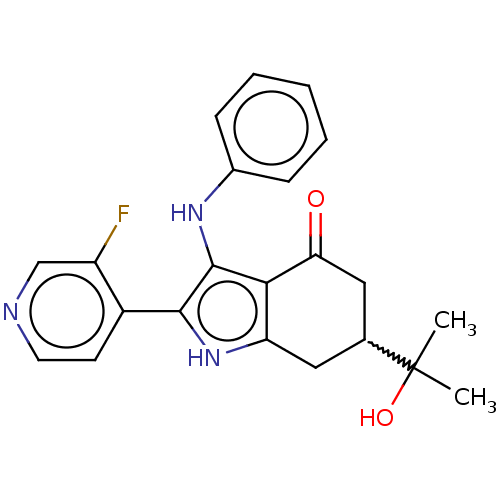
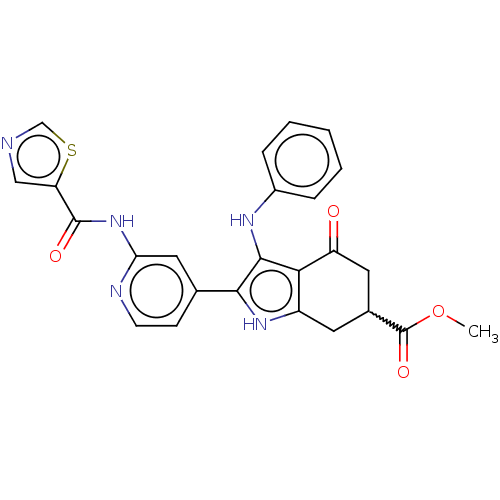
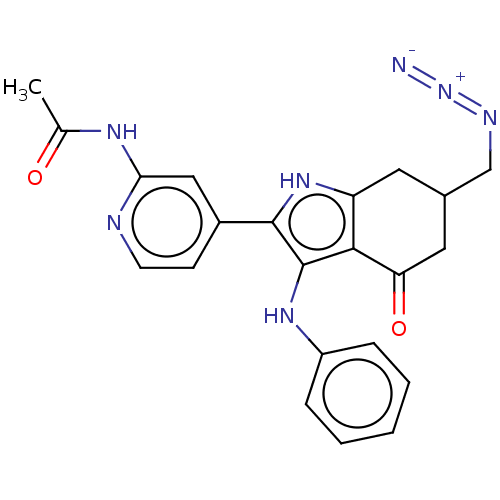
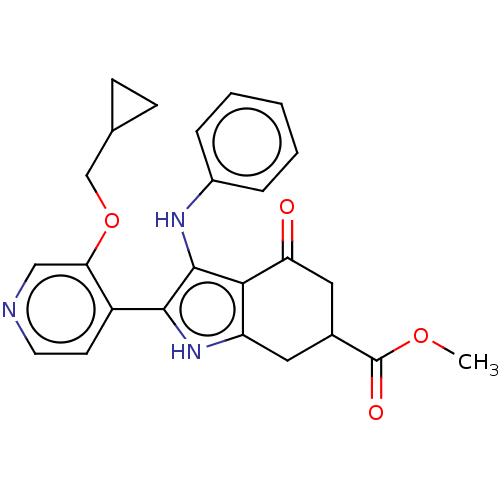
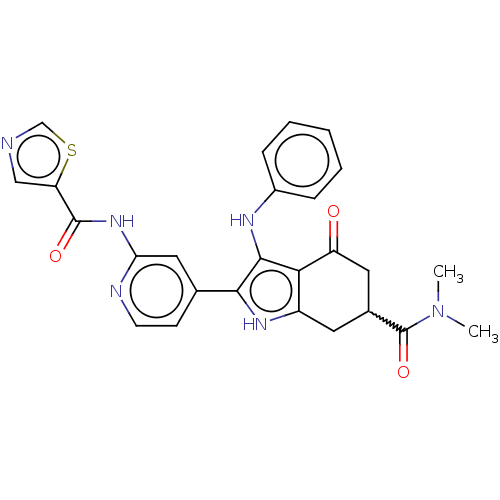
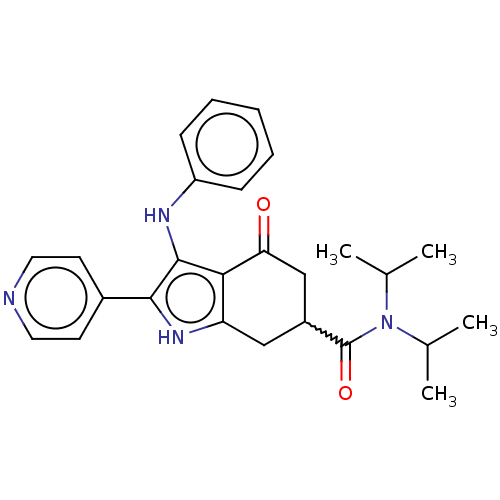
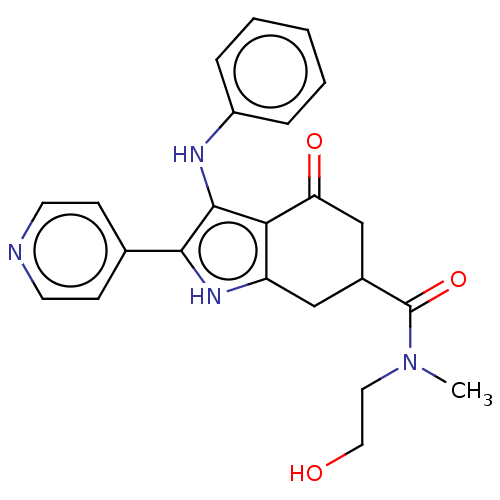
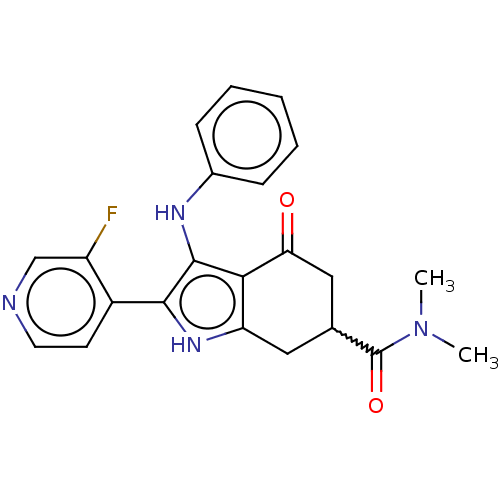
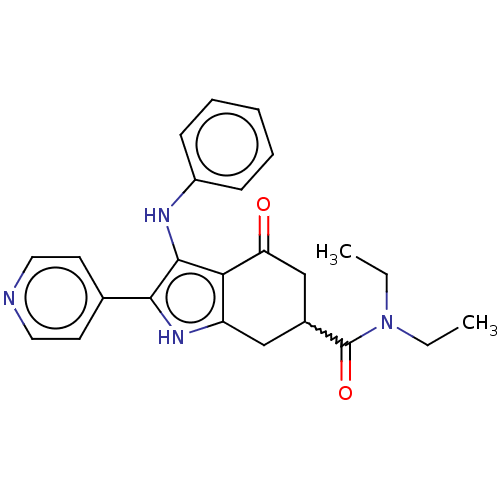
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe...More data for this Ligand-Target Pair
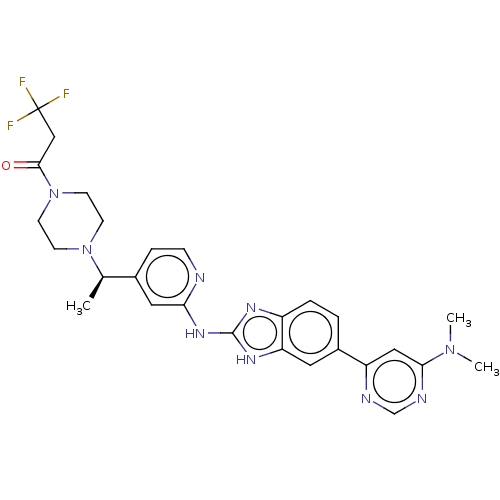
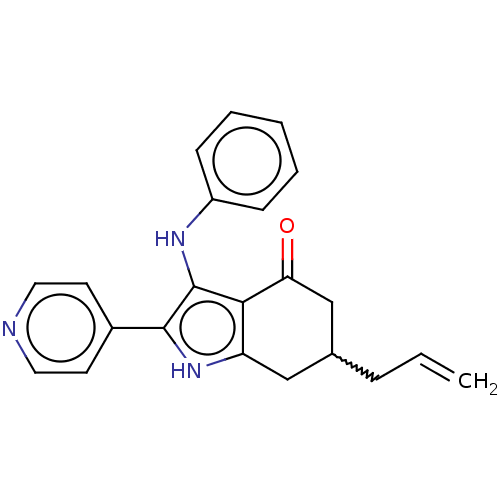
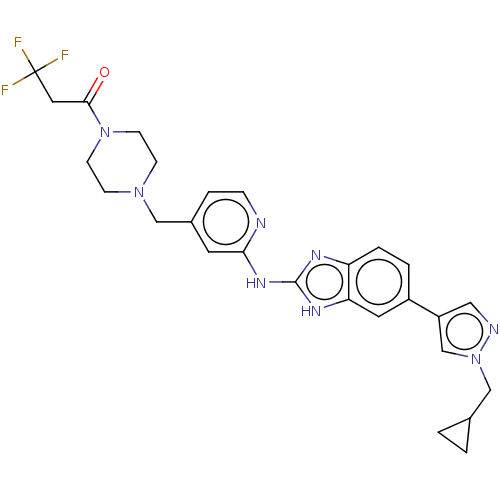
Affinity DataIC50: 0.700nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
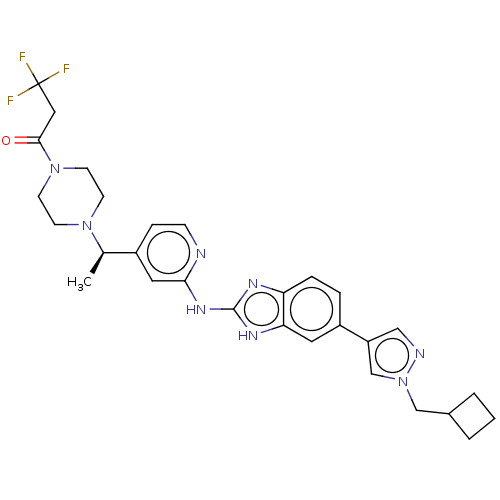
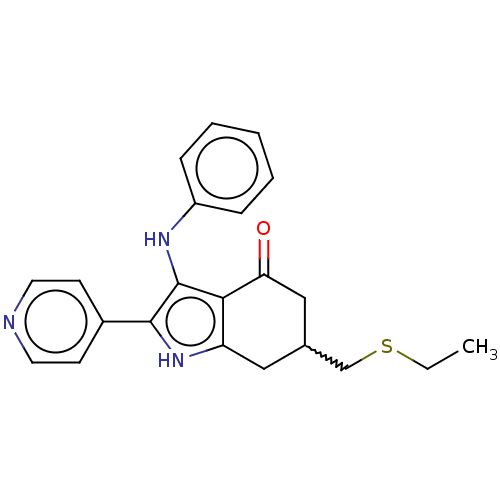
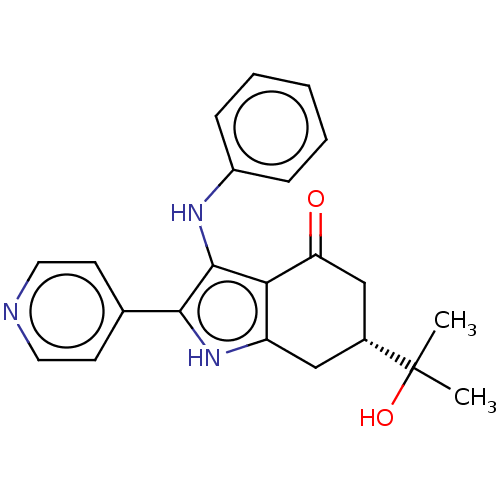
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe...More data for this Ligand-Target Pair
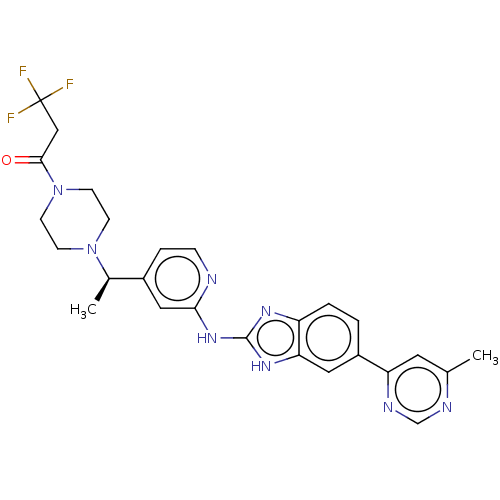
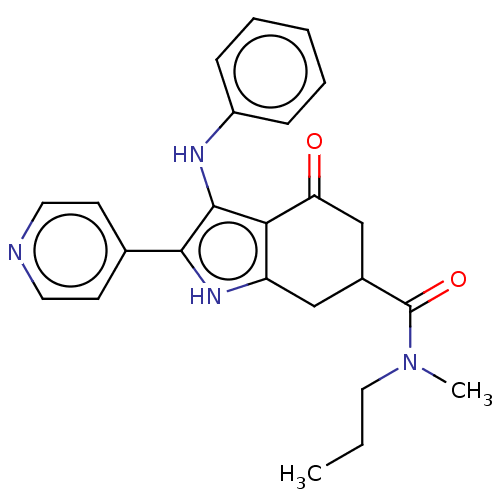
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
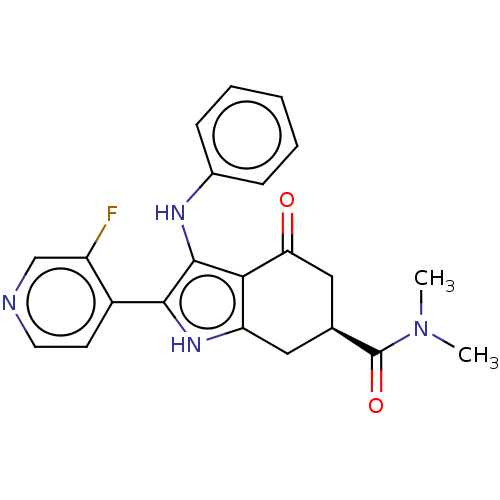
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant full length human His-tagged CDK9/CyclinT1 expressed in baculovirus expression system using biotin-labelled Ttds-YISPLKSPYK...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit epsilon(Homo sapiens (Human))
Bayer AG
Curated by ChEMBL
Bayer AG
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE...More data for this Ligand-Target Pair