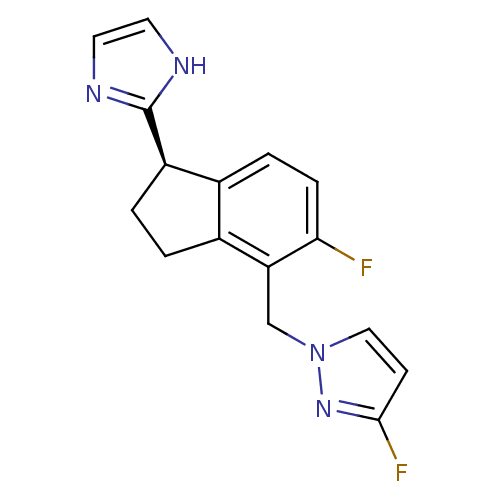
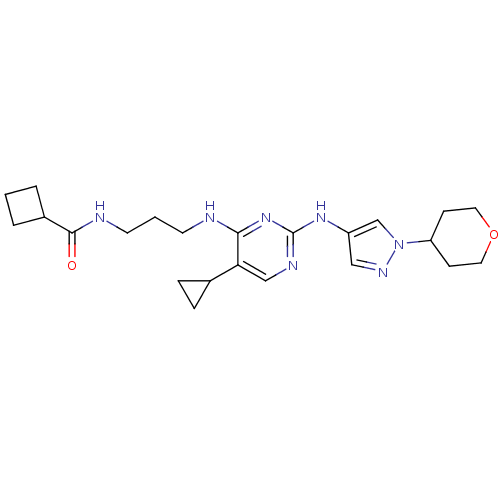
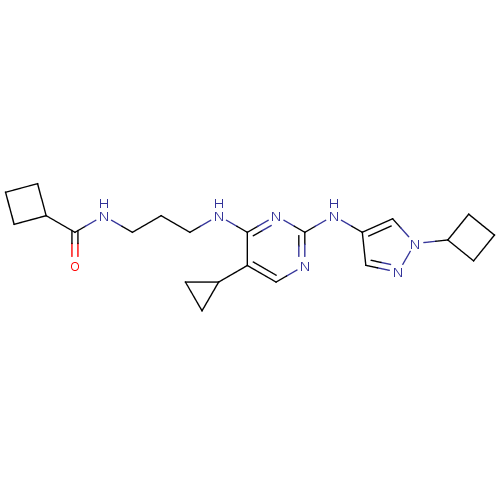
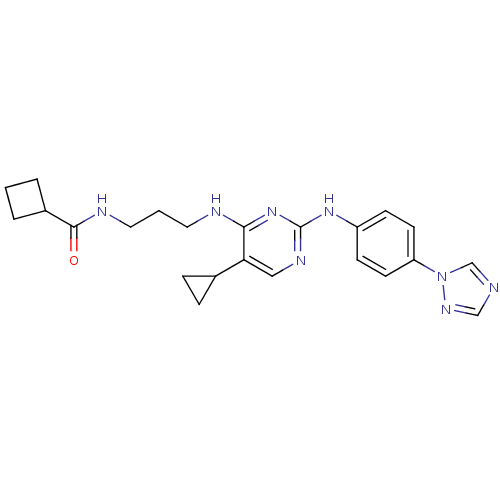
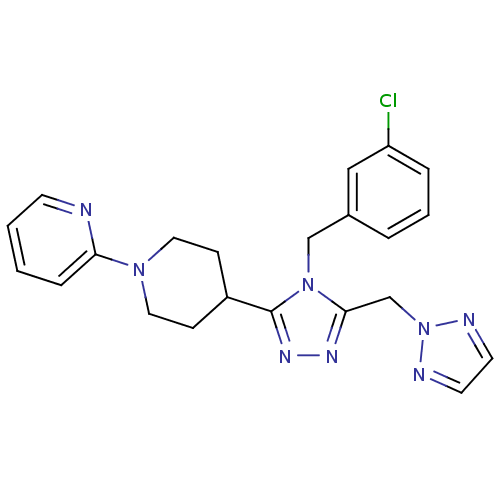
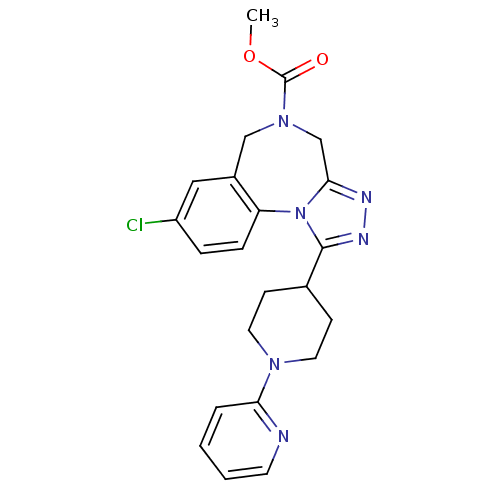
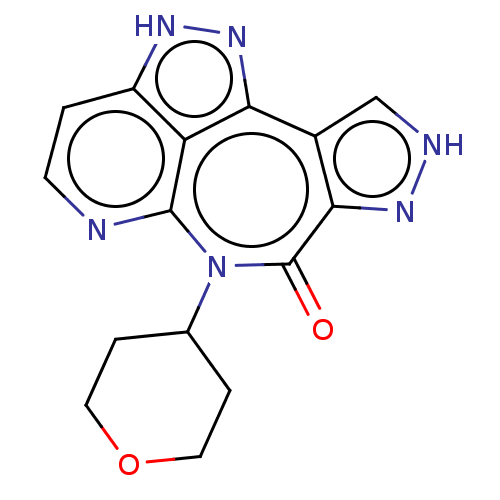
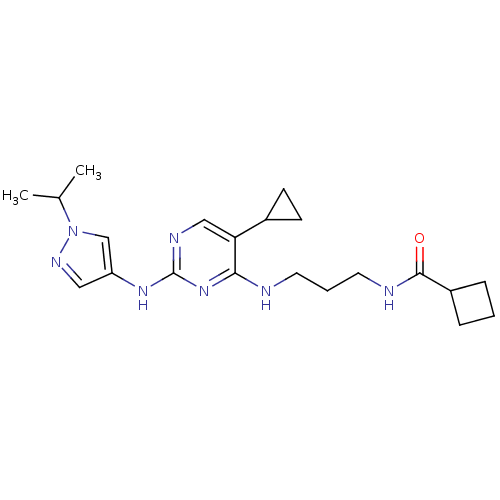
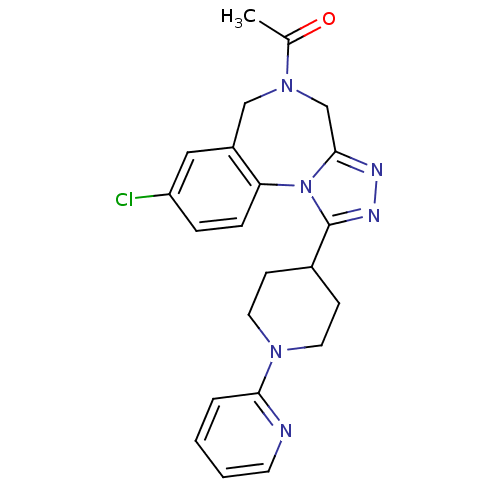
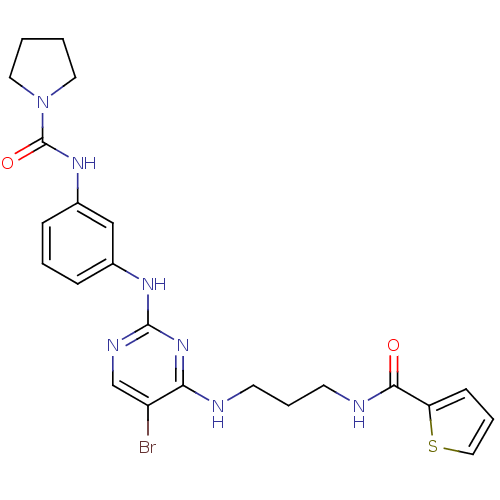
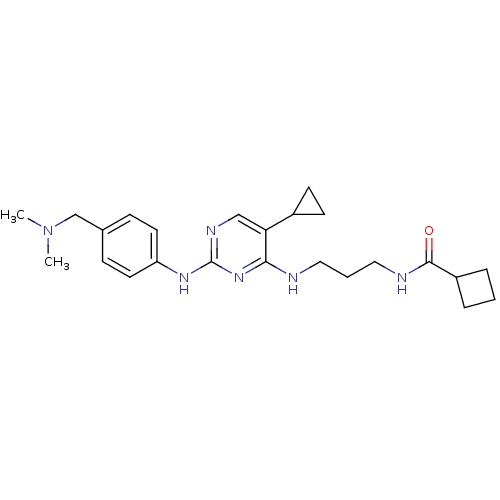
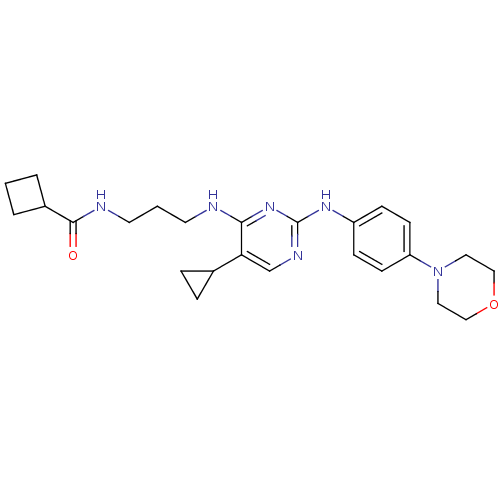
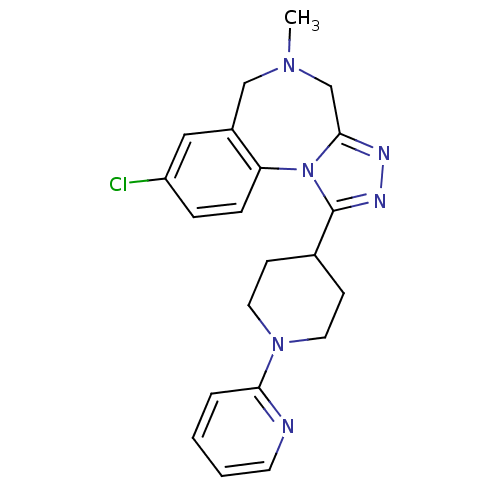
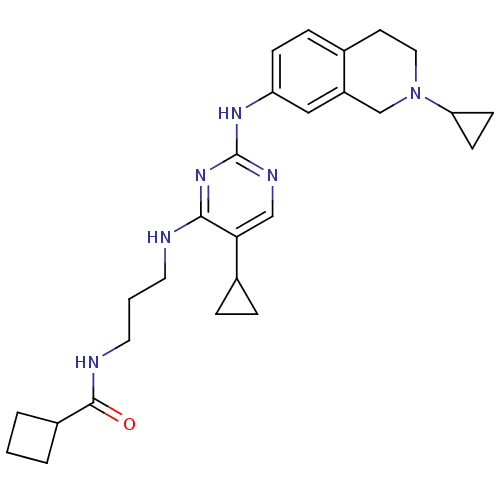
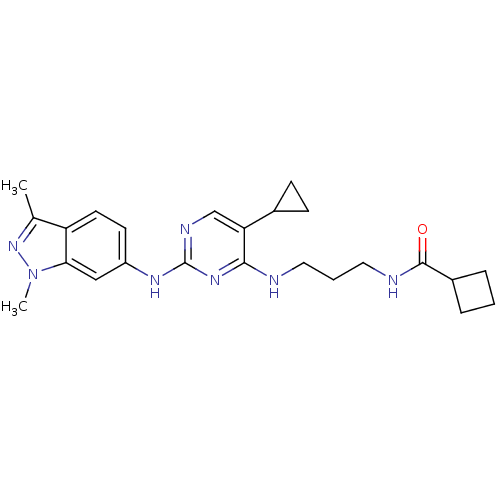
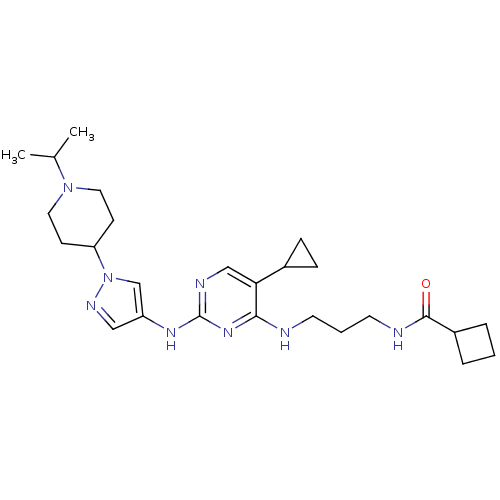
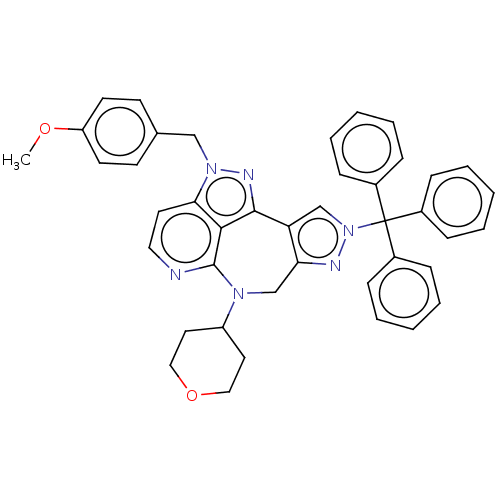
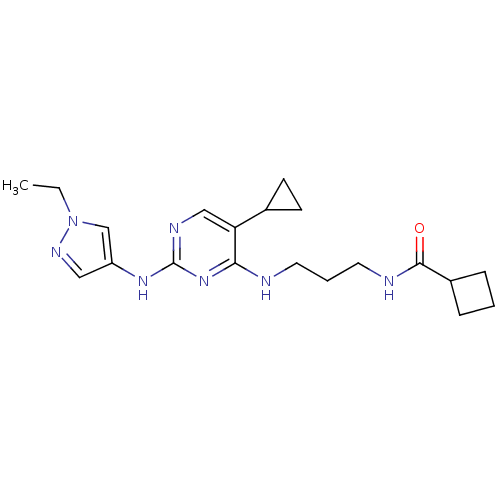
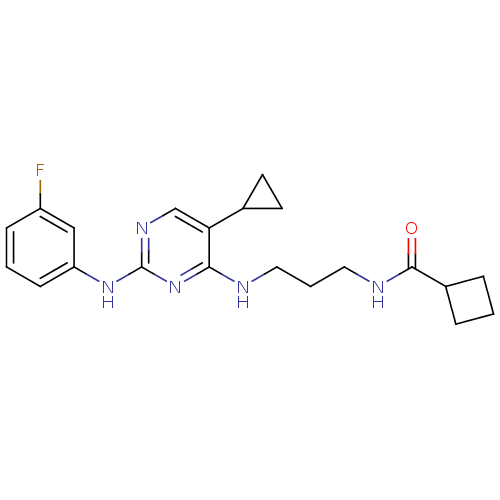
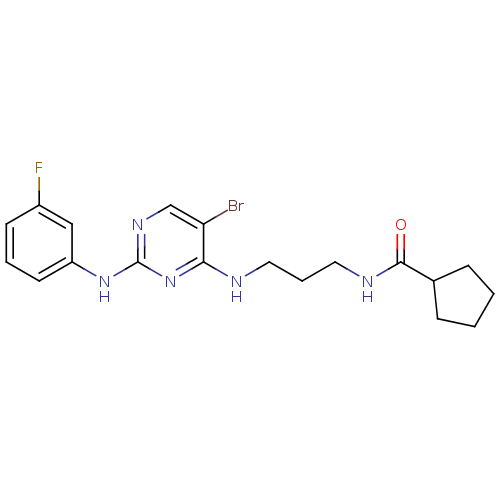
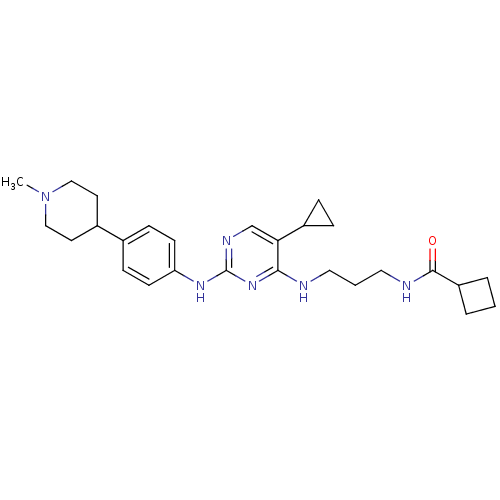
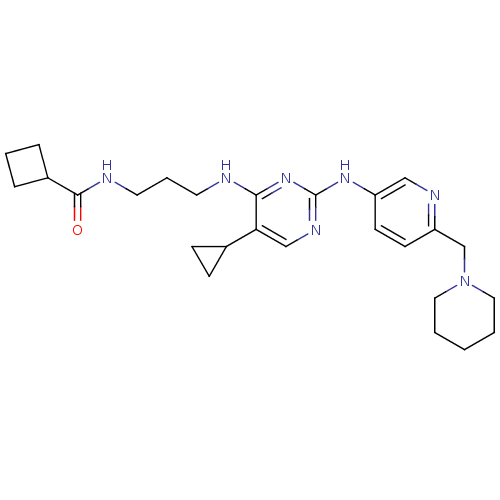
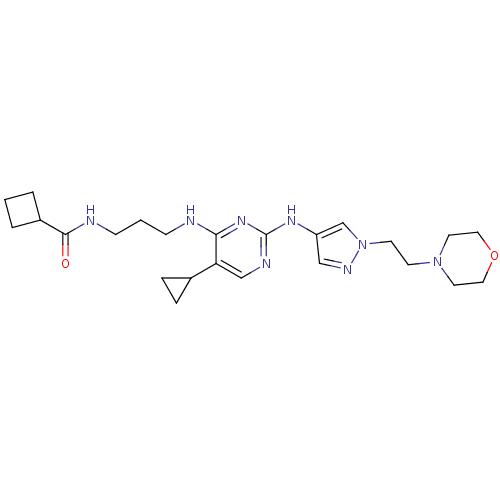
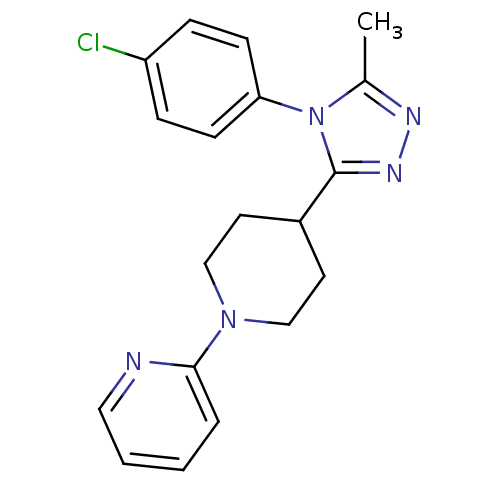
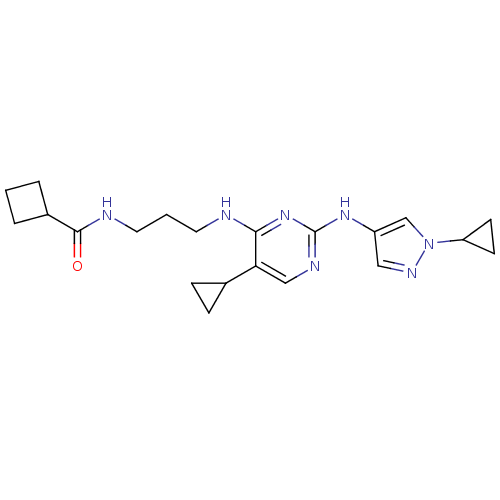
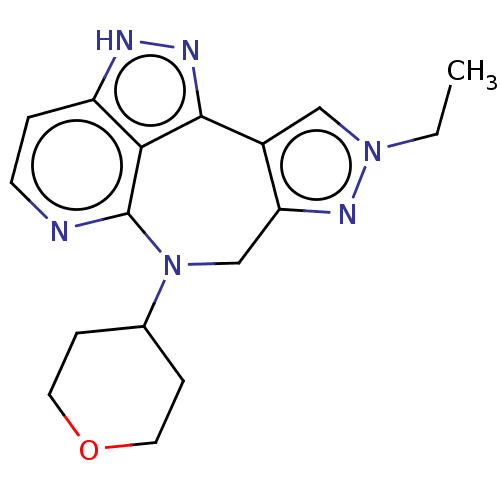
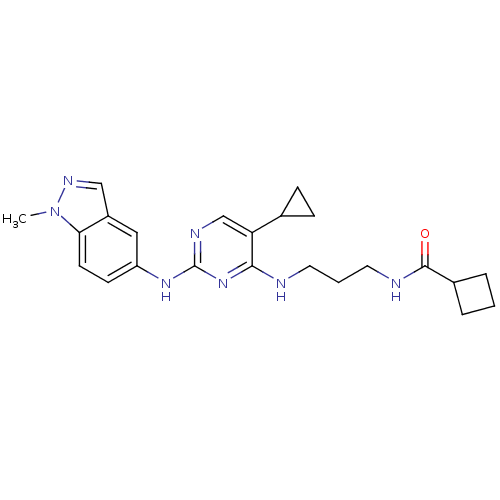
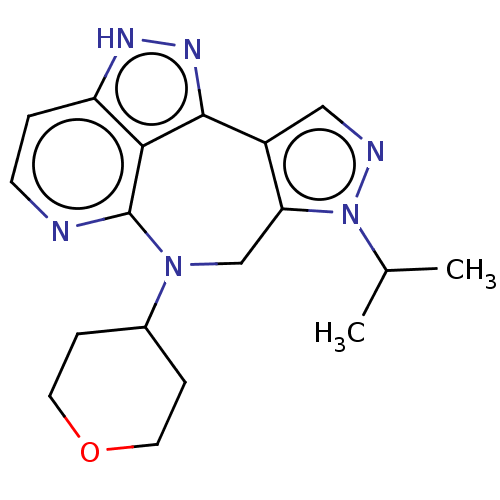
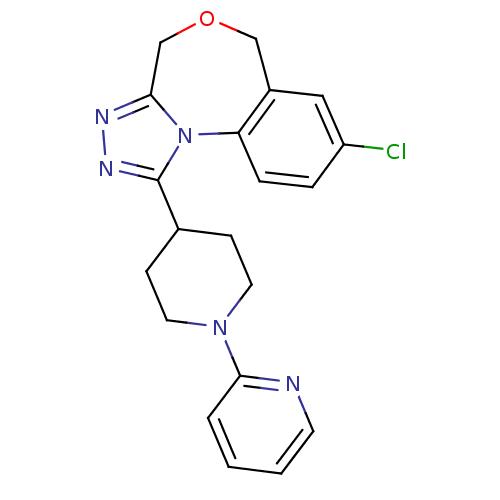
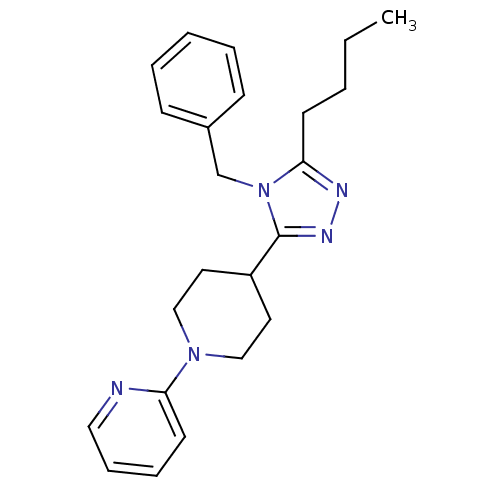
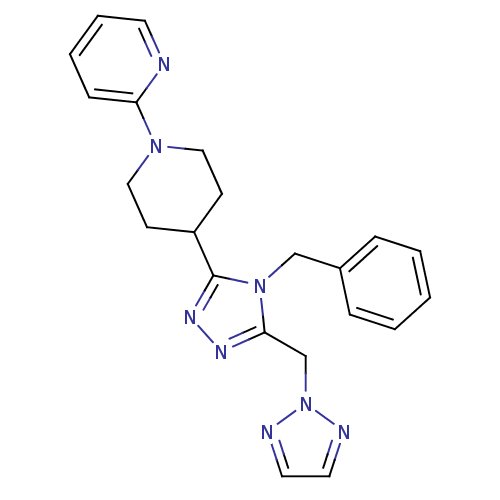
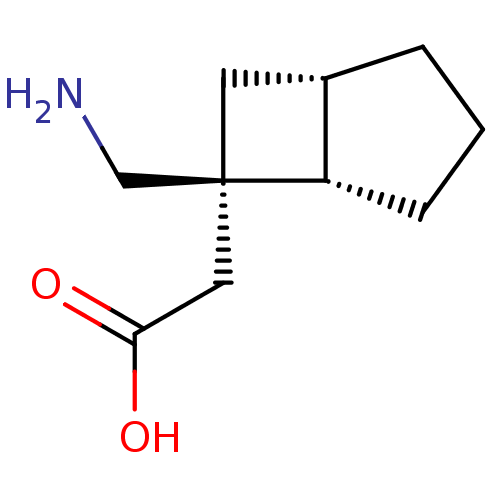
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
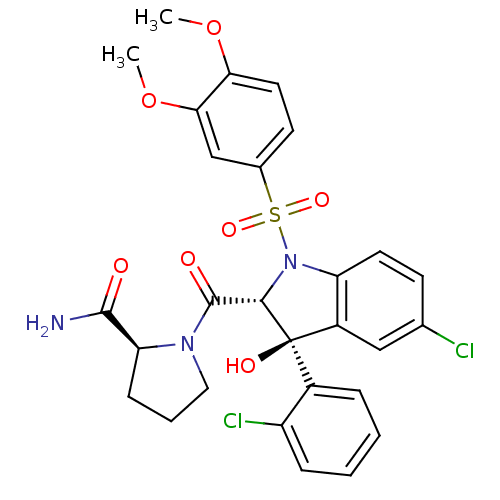
Affinity DataKi: 5nMAssay Description:Binding affinity to adrenergic alpha1A receptor (unknown origin)More data for this Ligand-Target Pair
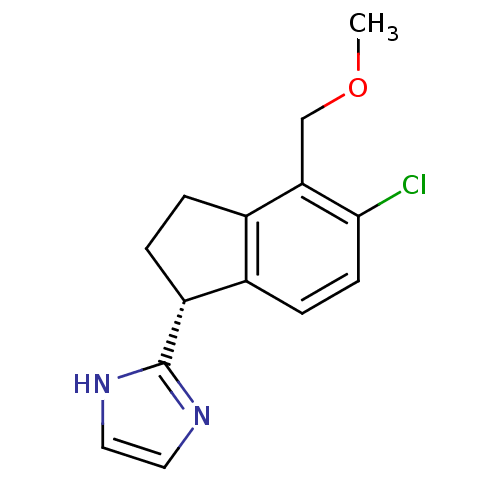
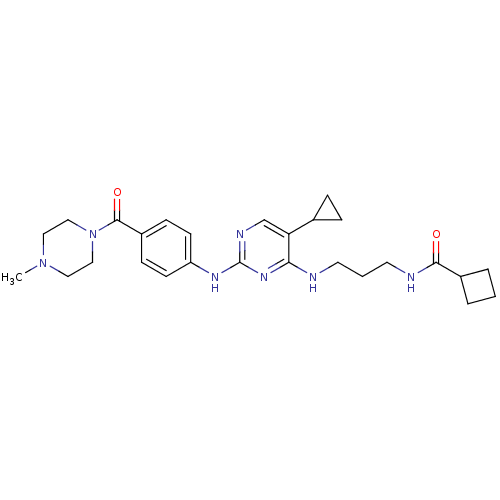
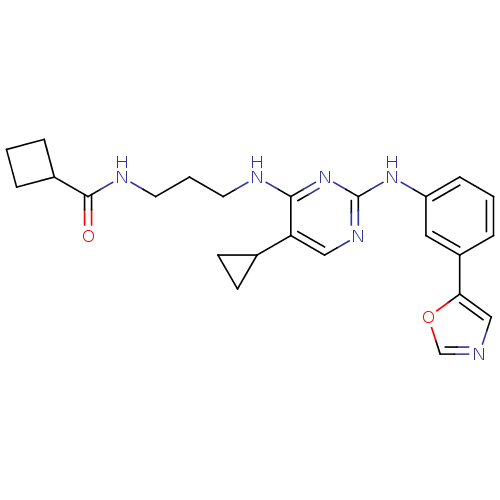
TargetAlpha-1A adrenergic receptor(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
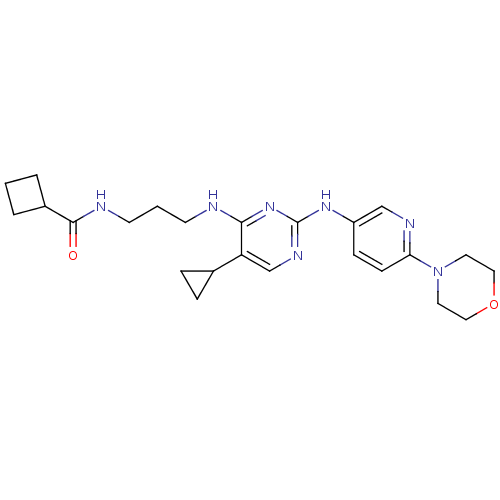
Affinity DataKi: 83nMAssay Description:Binding affinity to adrenergic alpha1A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
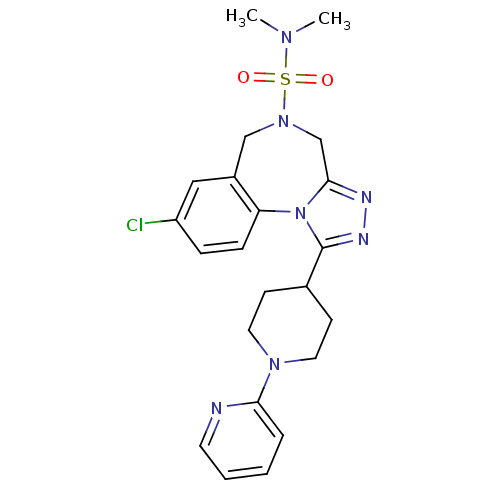
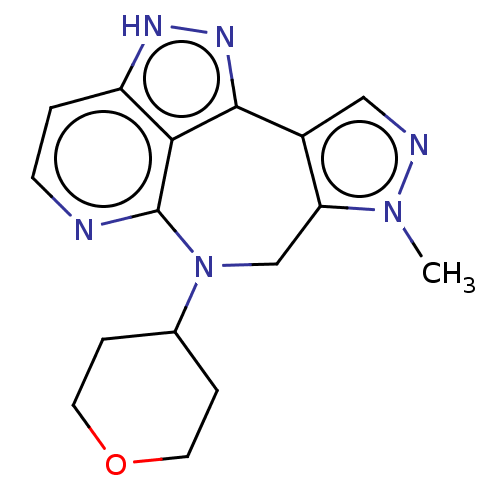
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 4nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
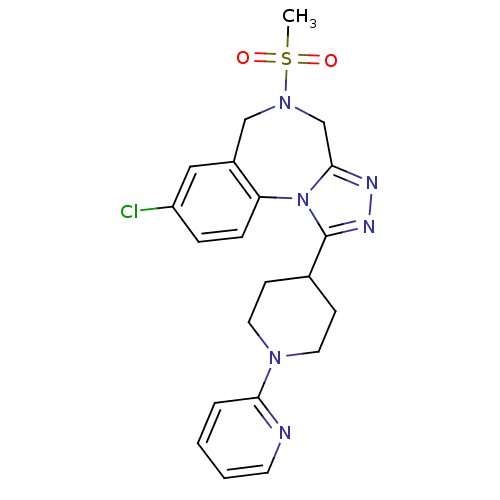
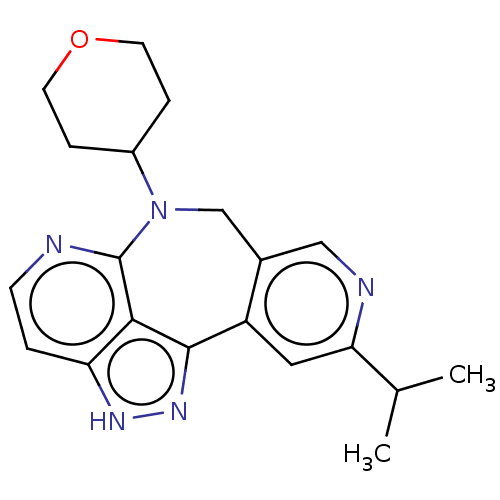
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 6nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 7nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 11nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 12nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
TargetMAP/microtubule affinity-regulating kinase 3(Homo sapiens (Human))
MRC Technology
Curated by ChEMBL
MRC Technology
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 13nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 15nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of TBK1 by radiometryMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
LIFEARC; SUZHOU YABAO PHARMACEUTICAL R&D CO., LTD.
US Patent
Affinity DataIC50: 16nMAssay Description:TTBK1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 1 mg/ml BSA, 10 mM DTT) is assayed against RRKDLHDDEEDEAMSITA ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human V1A receptor expressed in CHO cells assessed as inhibition of AVP-induced intracellular calcium release after 30 seconds...More data for this Ligand-Target Pair
TargetVoltage-dependent calcium channel alpha-2 delta subunit(Sus scrofa)
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Displacement of [3H]gabapentin from calcium channel alpha2delta in pig cerebral cortex membrane after 30 minsMore data for this Ligand-Target Pair