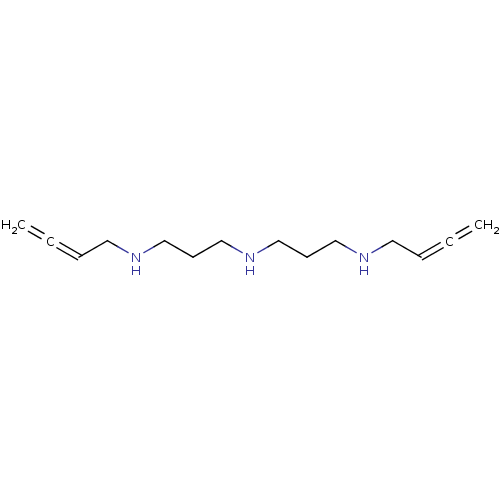
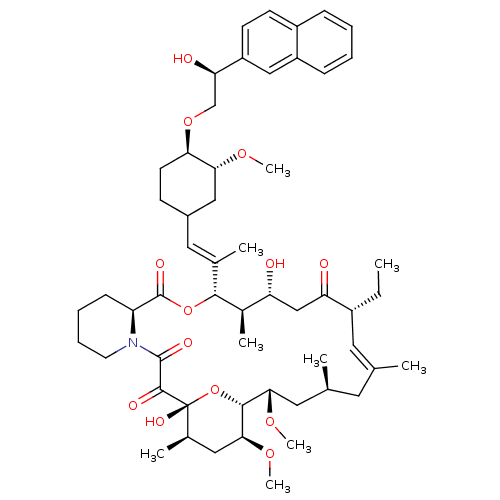
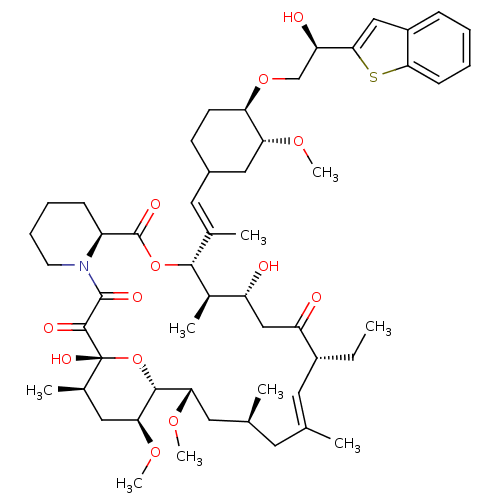
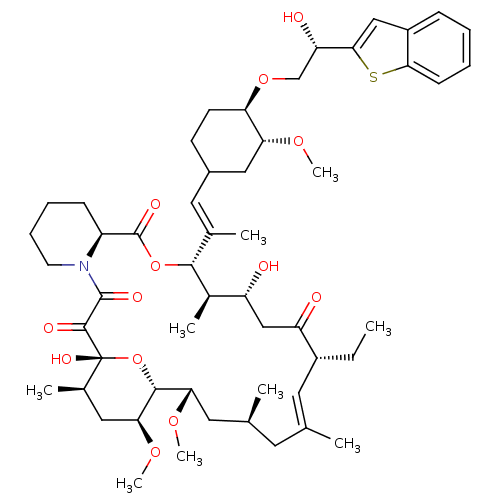
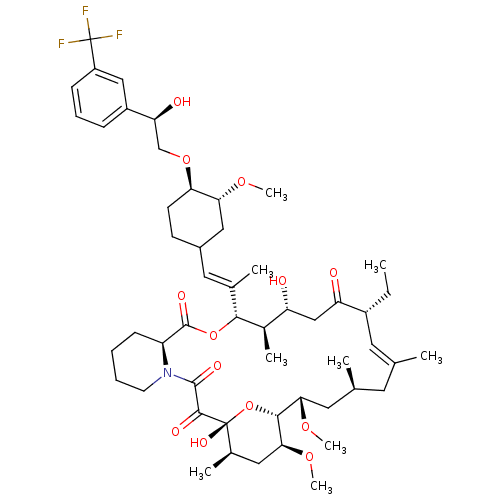
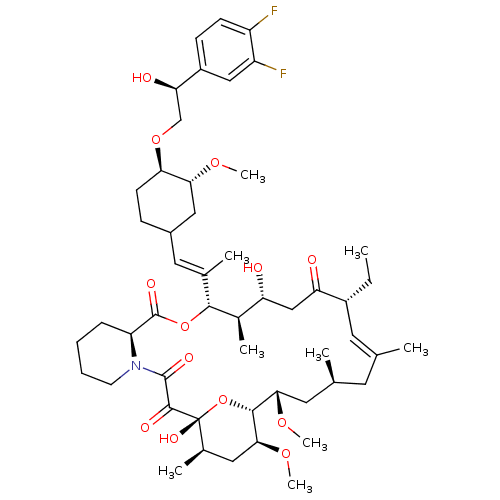
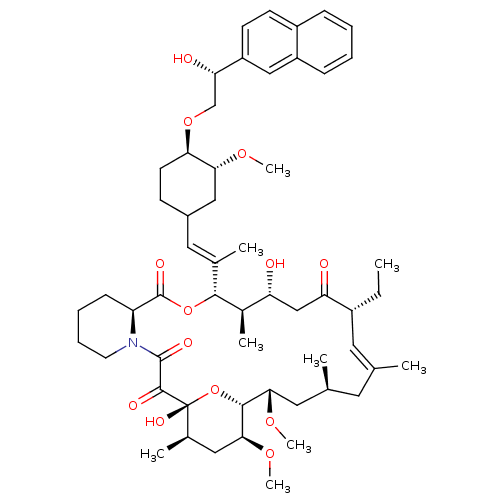
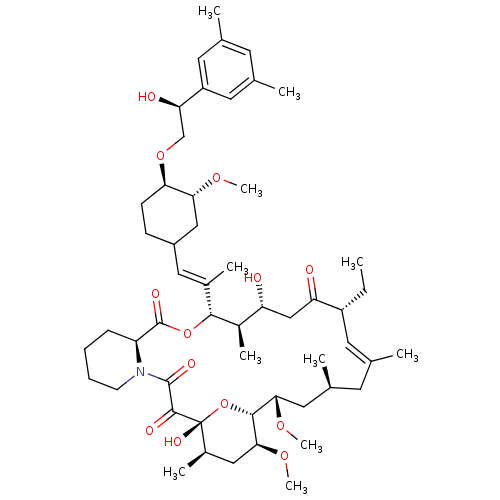
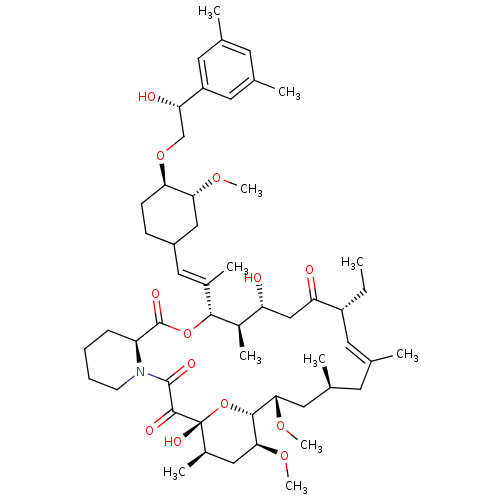
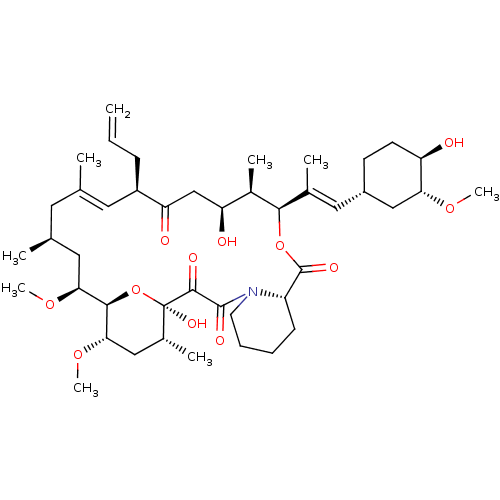
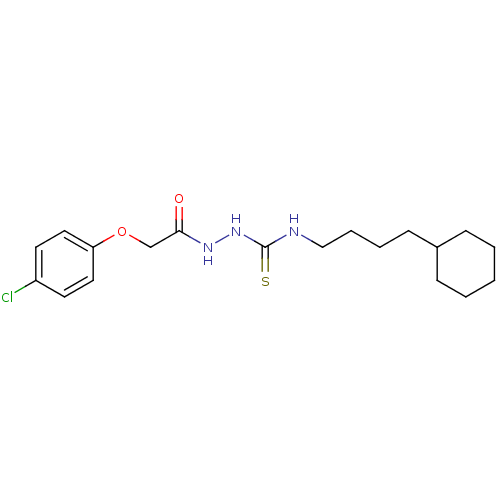
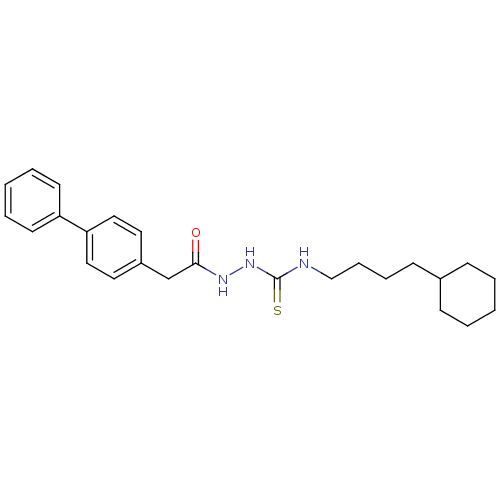
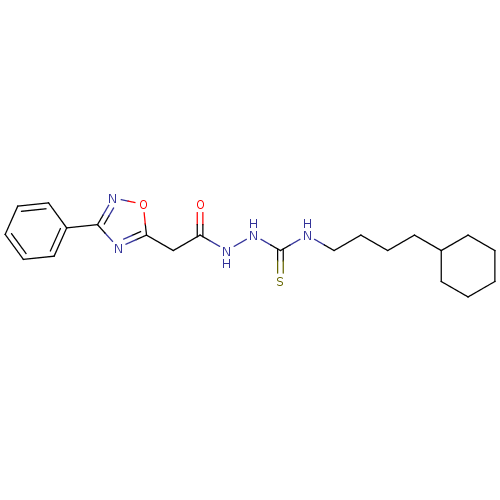
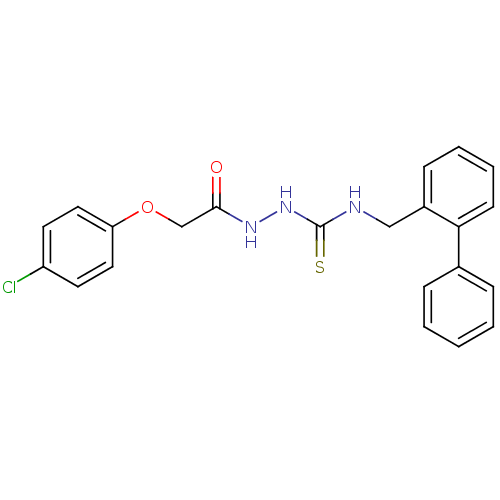
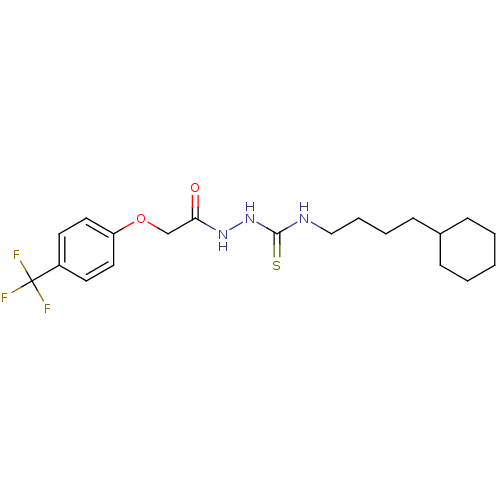
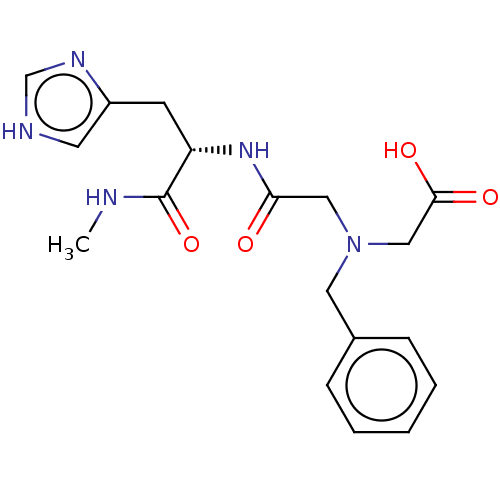
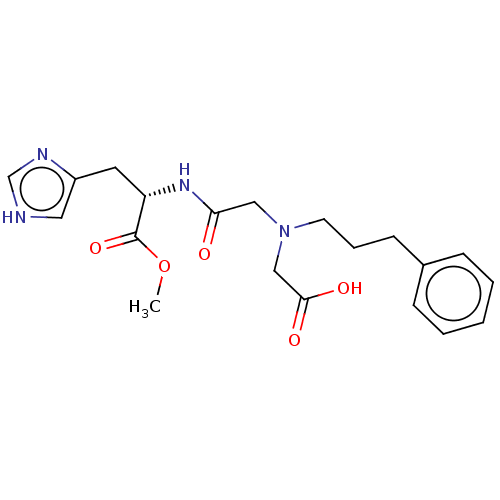
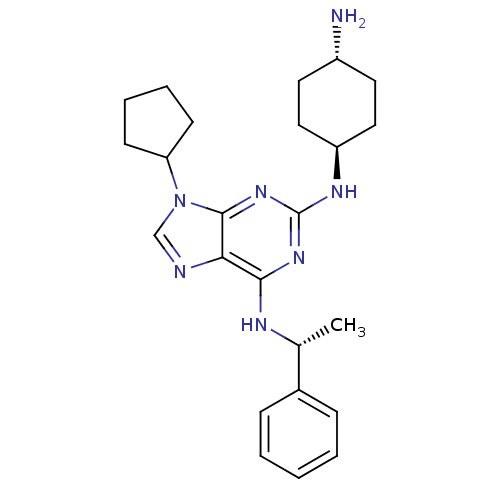
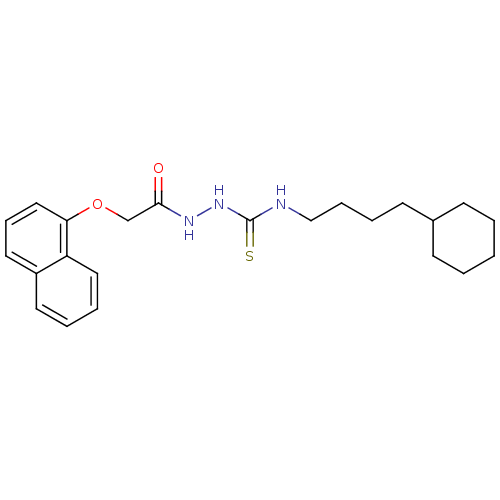
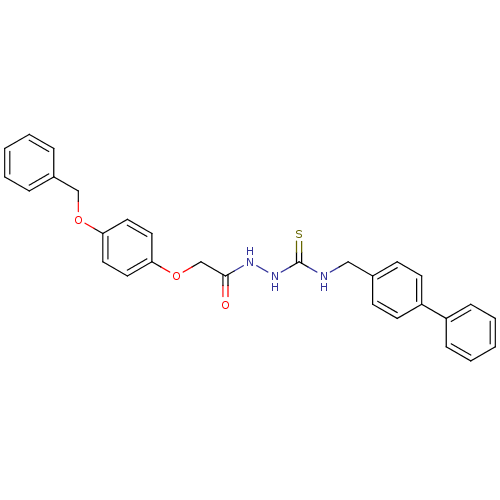
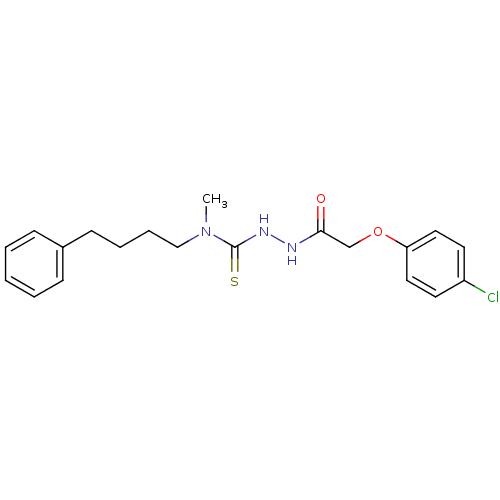
TargetPeptidyl-prolyl cis-trans isomerase A(Homo sapiens (Human))
Southern Research Institute
Curated by ChEMBL
Southern Research Institute
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activityMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase A(Homo sapiens (Human))
Southern Research Institute
Curated by ChEMBL
Southern Research Institute
Curated by ChEMBL
Affinity DataKi: 2.11nMAssay Description:Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activityMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase A(Homo sapiens (Human))
Southern Research Institute
Curated by ChEMBL
Southern Research Institute
Curated by ChEMBL
Affinity DataKi: 9.79nMAssay Description:Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activityMore data for this Ligand-Target Pair
TargetPeroxisomal N(1)-acetyl-spermine/spermidine oxidase(Mus musculus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Tested for in vitro binding affinity against polyamine oxidase after intraperitoneal administration in the rat liverMore data for this Ligand-Target Pair
TargetPeroxisomal N(1)-acetyl-spermine/spermidine oxidase(Mus musculus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: 2.50E+3nMAssay Description:Tested for in vitro binding affinity against polyamine oxidase after intraperitoneal administration in the rat liverMore data for this Ligand-Target Pair
TargetPeroxisomal N(1)-acetyl-spermine/spermidine oxidase(Mus musculus)
Merrell Dow Research Institute
Curated by ChEMBL
Merrell Dow Research Institute
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Tested for in vitro binding affinity against polyamine oxidase after intraperitoneal administration in the rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human NEP-mediated amyloid beta hydrolysisMore data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.90nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.20nMAssay Description:Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human ACE-mediated amyloid beta hydrolysisMore data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Concentration required for inhibition of serine/threonine protein phosphatase calcineurin (CAN)More data for this Ligand-Target Pair
TargetProtein phosphatase 3 catalytic subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibitory activity against Calcineurin (CaN phosphatase)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 24nMpH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 48nMpH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 52nMpH: 8.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 70nMpH: 8.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 226nMpH: 8.0 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human recombinant IDE-mediated amyloid beta (16 to 23) hydrolysis using ATTO 655-Cys-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Trp as substrate p...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 4(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 610nMAssay Description:Inhibition of human recombinant ADAMTS-4 using QTVTWPDMELPLPRNITEGEARGSVIL-TVKPIFEVSPSPL(biotinyl)K as substrate incubated for 10 mins prior to subst...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 4(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 630nMAssay Description:Inhibition of human recombinant ADAMTS-4 using QTVTWPDMELPLPRNITEGEARGSVIL-TVKPIFEVSPSPL(biotinyl)K as substrate incubated for 10 mins prior to subst...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 720nMpH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 4(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 730nMAssay Description:Inhibition of human recombinant ADAMTS-4 using QTVTWPDMELPLPRNITEGEARGSVIL-TVKPIFEVSPSPL(biotinyl)K as substrate incubated for 10 mins prior to subst...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 780nMpH: 7.5 T: 2°CAssay Description:The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 4(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 790nMAssay Description:Inhibition of human recombinant ADAMTS-4 using QTVTWPDMELPLPRNITEGEARGSVIL-TVKPIFEVSPSPL(biotinyl)K as substrate incubated for 10 mins prior to subst...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 4(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of human recombinant ADAMTS-4 using QTVTWPDMELPLPRNITEGEARGSVIL-TVKPIFEVSPSPL(biotinyl)K as substrate incubated for 10 mins prior to subst...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair
TargetA disintegrin and metalloproteinase with thrombospondin motifs 5(Homo sapiens (Human))
INSERM
Curated by ChEMBL
INSERM
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of human recombinant ADAMTS-5 using ARGSVILTV-KPIFEVSPSPL(biotinyl)K as substrate incubated 10 mins prior to substrate addition measured a...More data for this Ligand-Target Pair


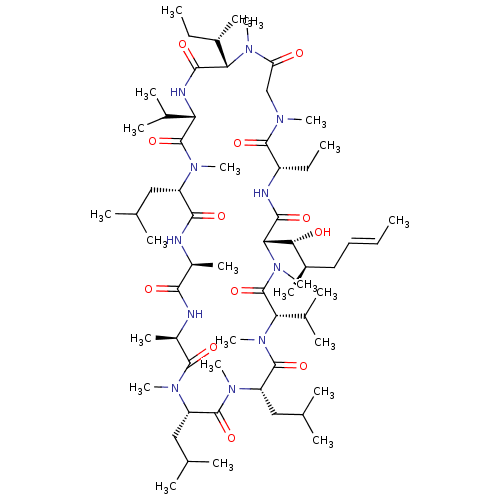

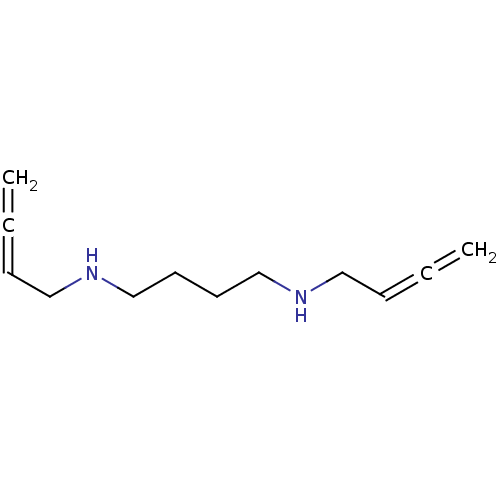
 3D Structure (crystal)
3D Structure (crystal)