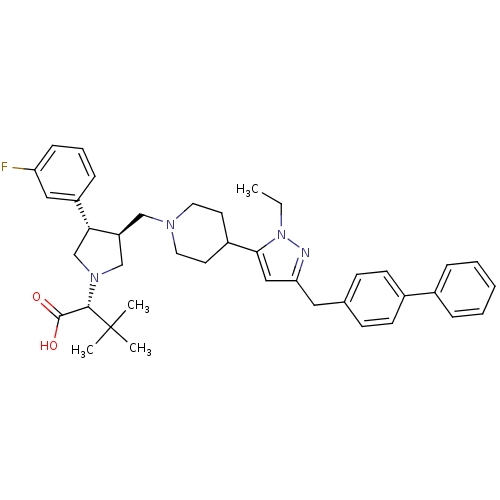
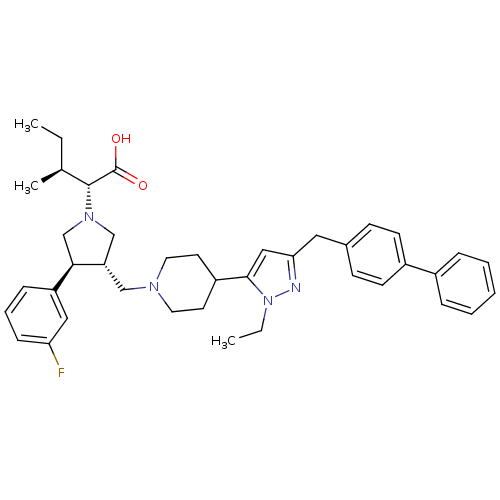
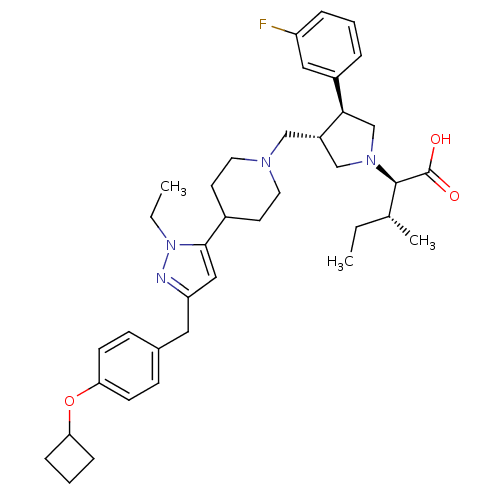
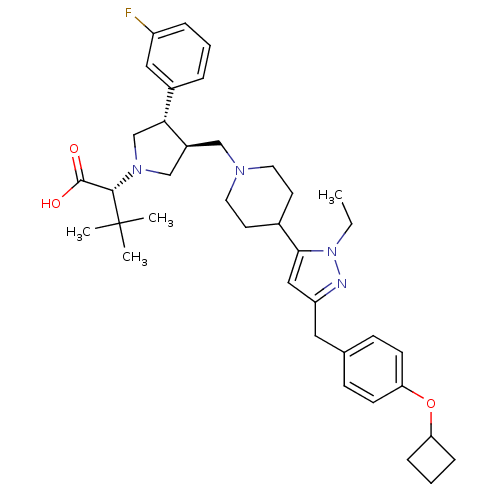
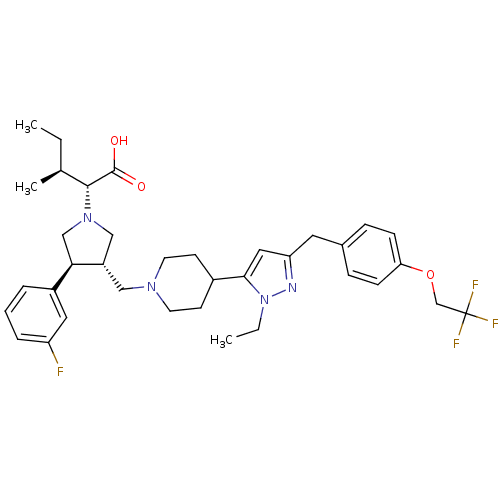
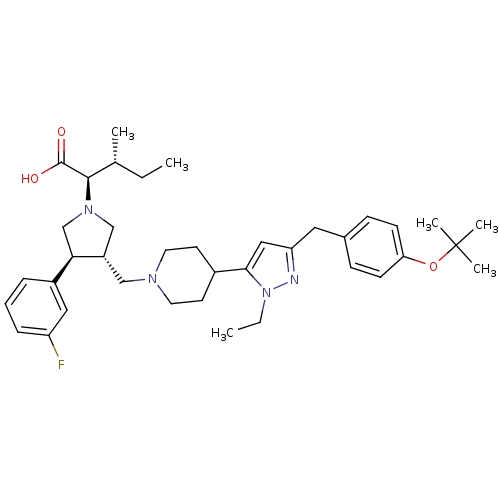
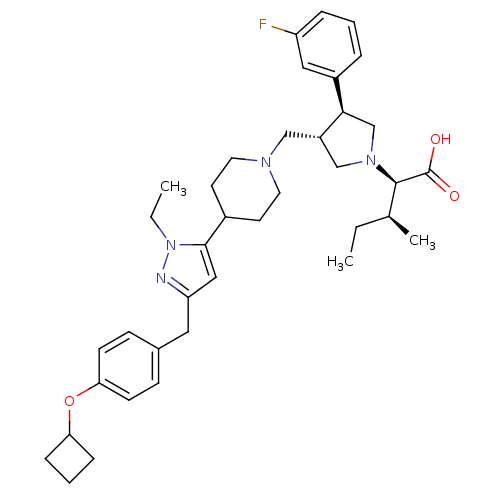
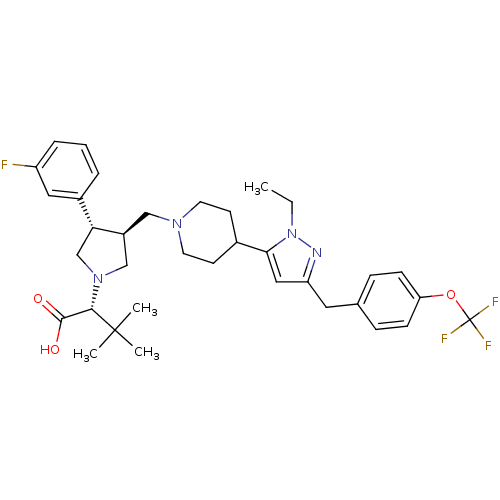
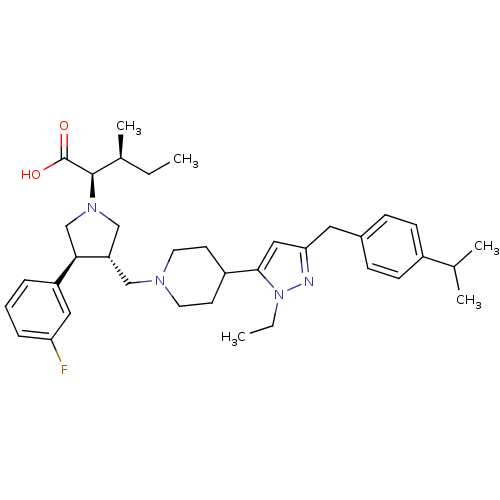
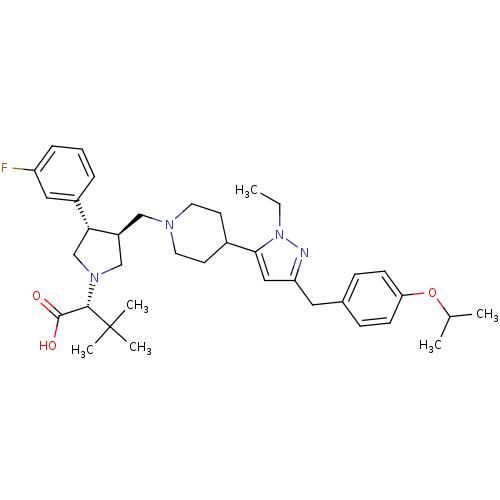
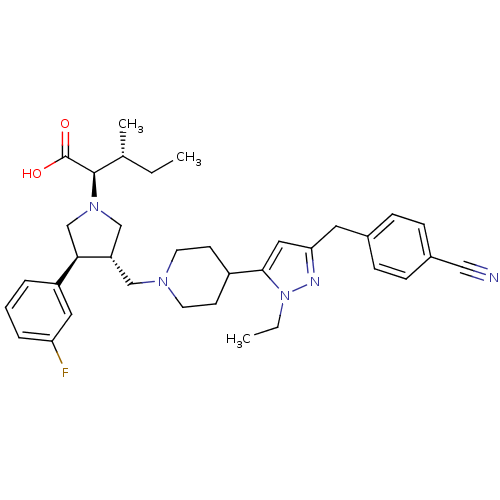
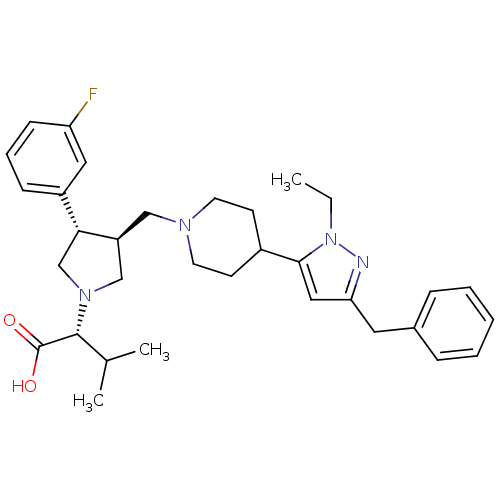
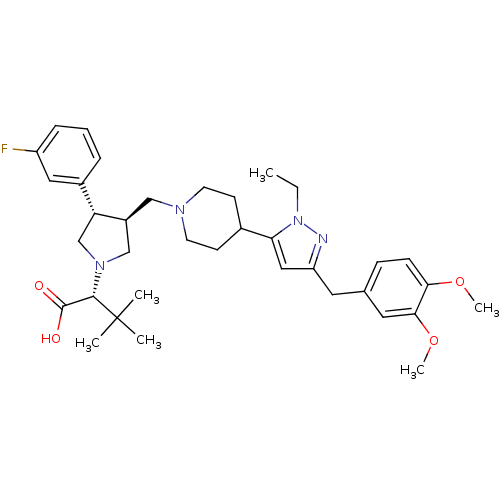
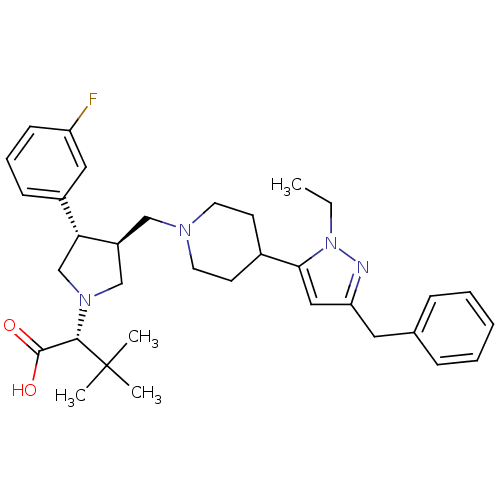
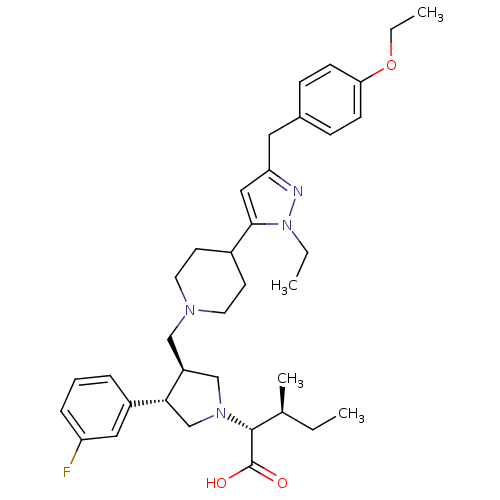
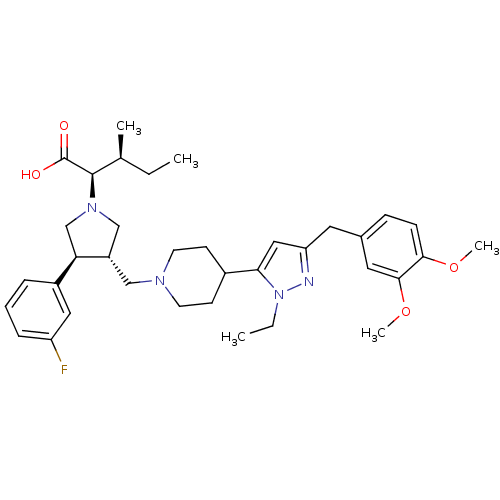
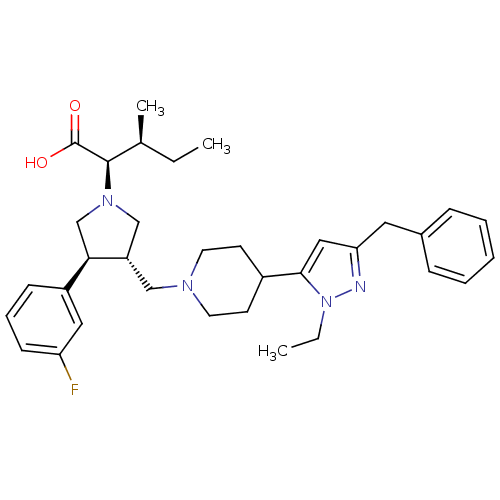
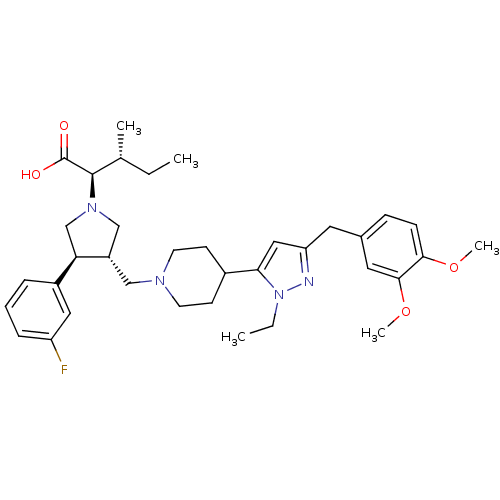
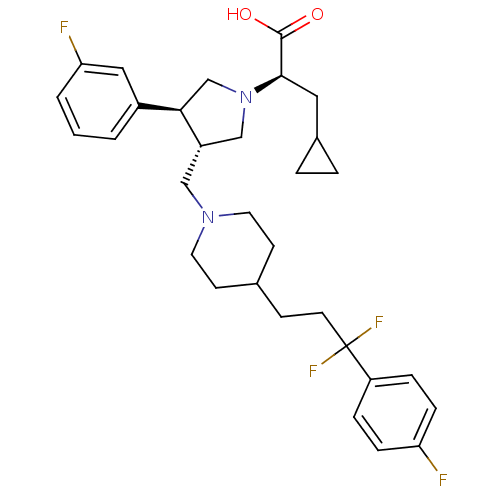
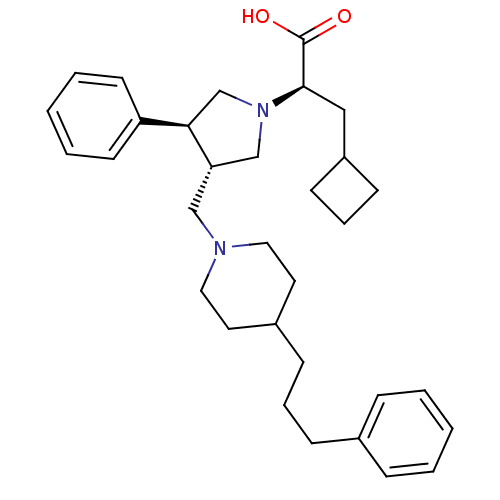
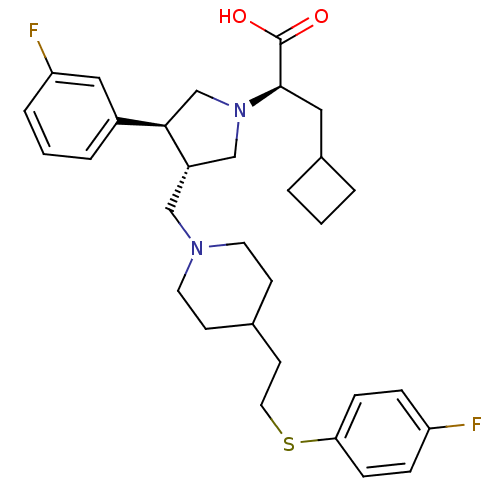
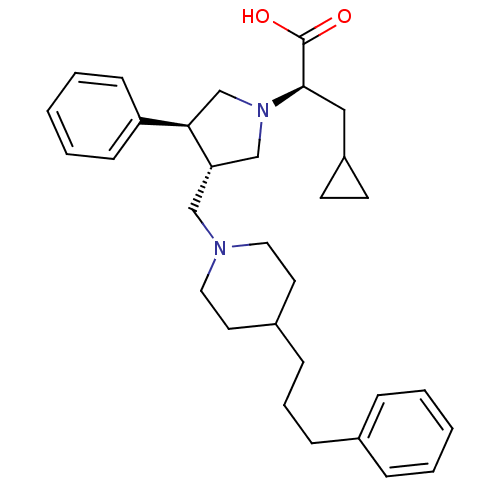
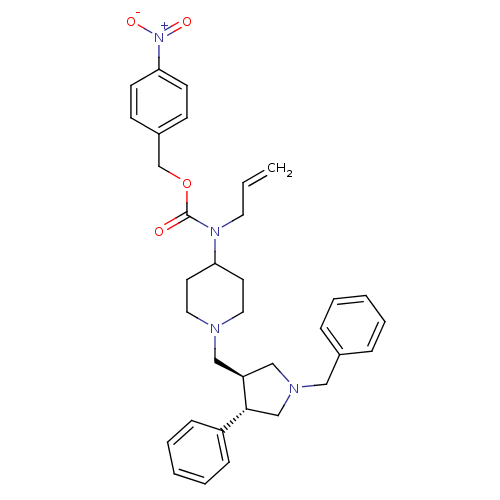
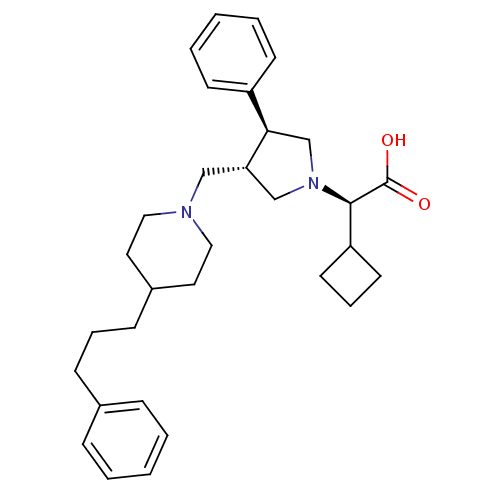
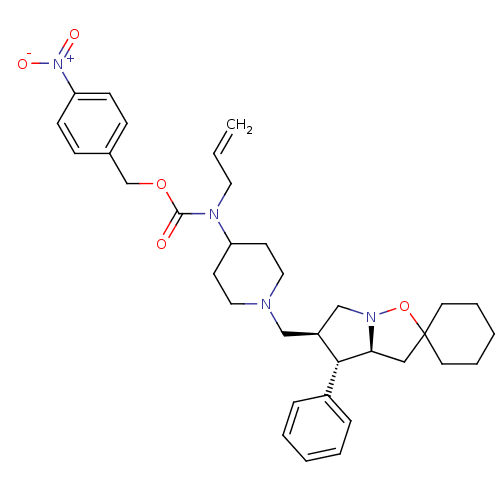
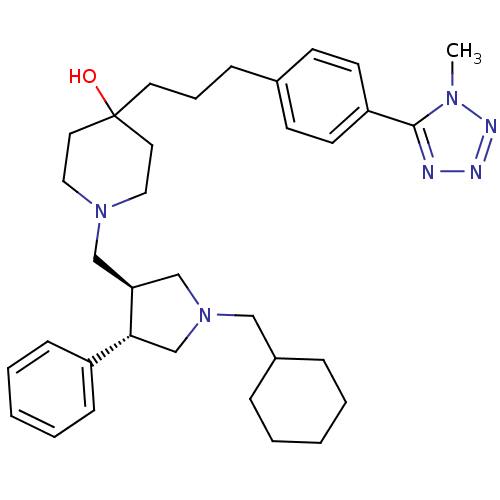
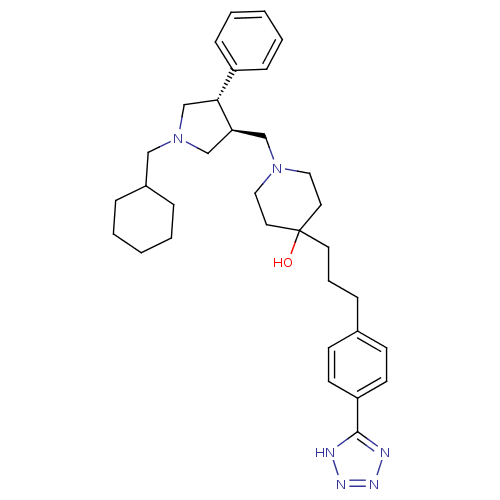
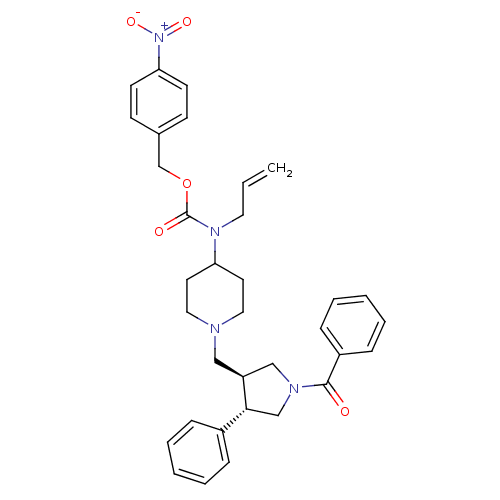
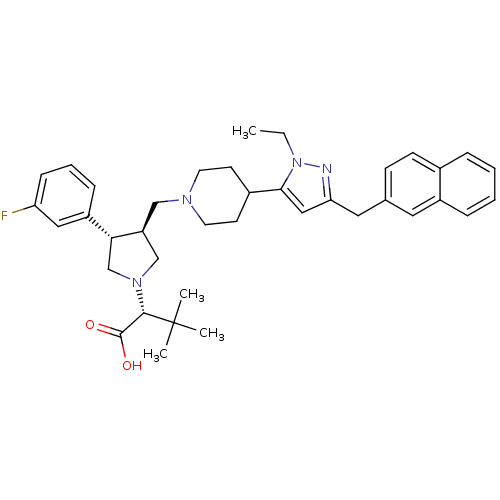
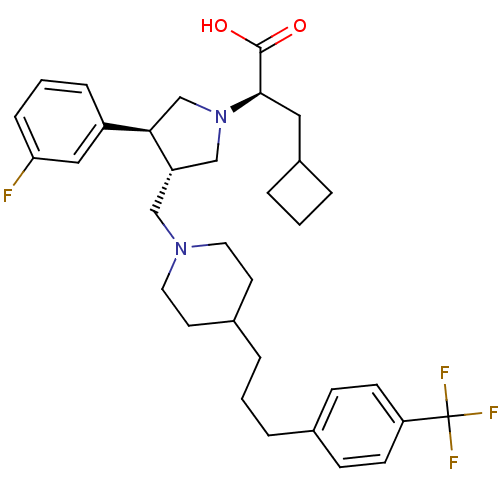
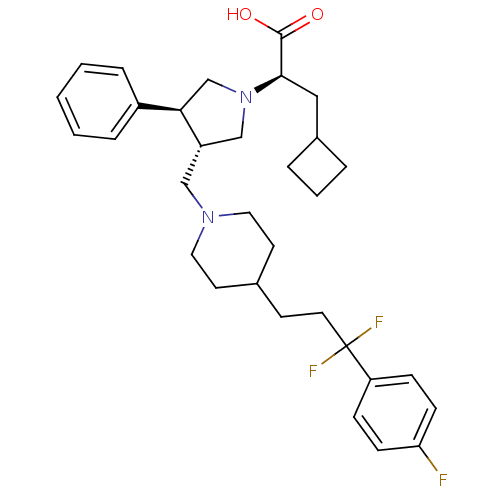
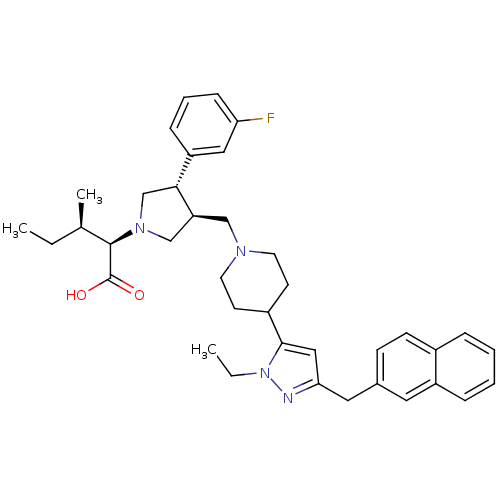
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.50E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.80E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.60E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.70E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 9.60E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.70E+4nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membraneMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membraneMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membraneMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranesMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Ability to displace [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranesMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranesMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]-labeled MIP-1alpha from the C-C chemokine receptor type 5 expressed on CHO cell membranesMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:In vitro binding affinity at CCR5 receptor in the presence of [125I]-MIP-1 alpha.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration, binding towards C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand expressed on CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human CX3C chemokine receptor 5 from GP120-membrane-based assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membraneMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membraneMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cellMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligandMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligandMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.290nMAssay Description:Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligandMore data for this Ligand-Target Pair