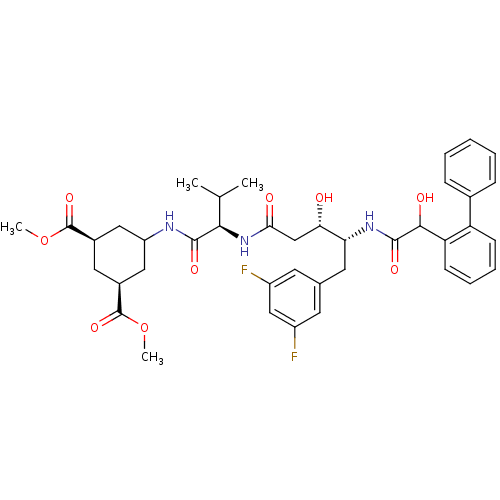
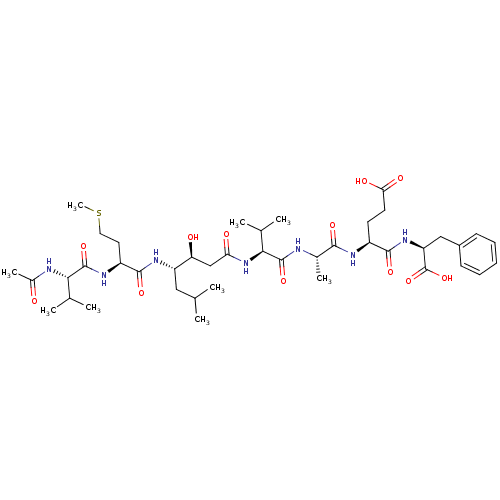
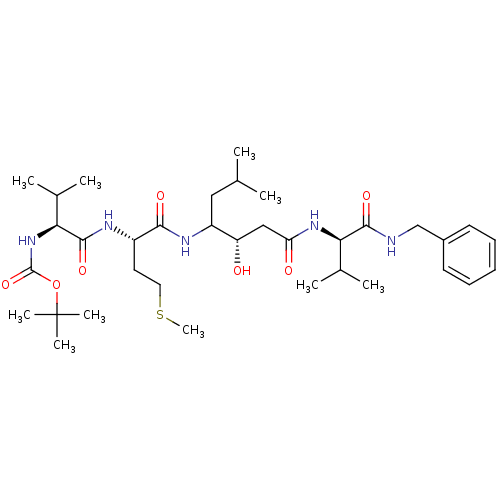
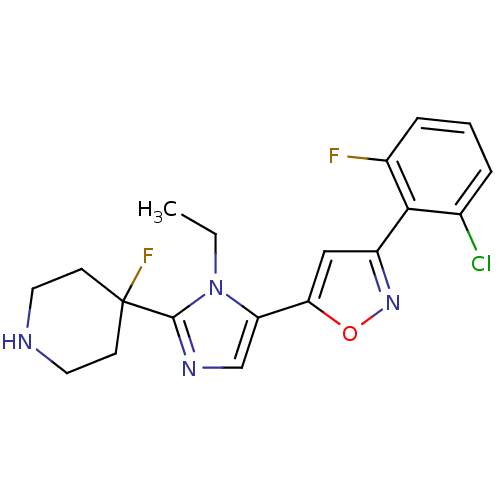
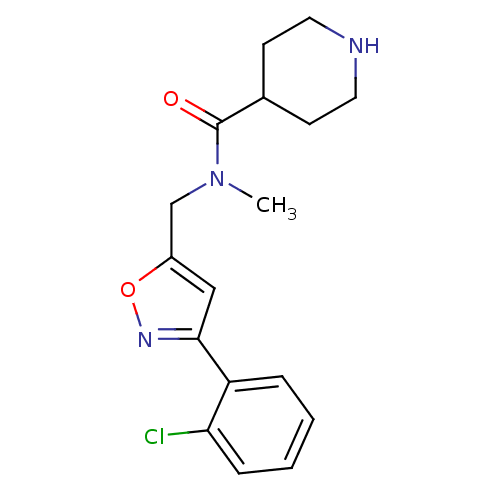
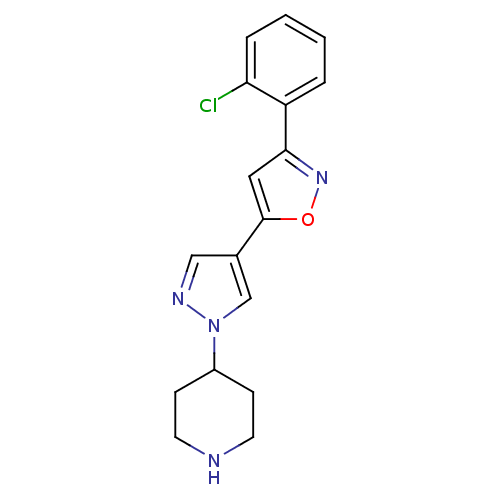
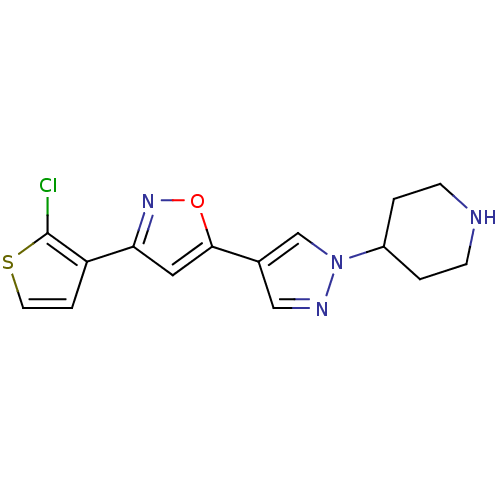
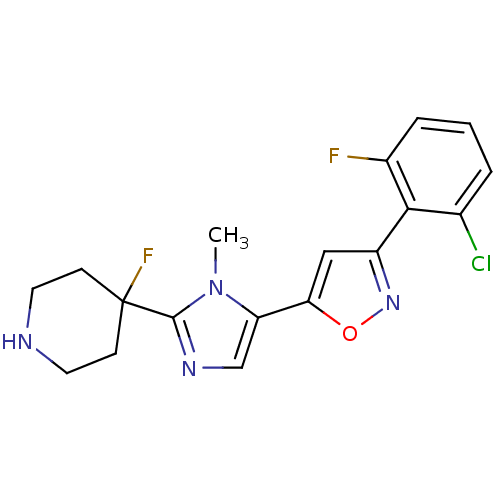
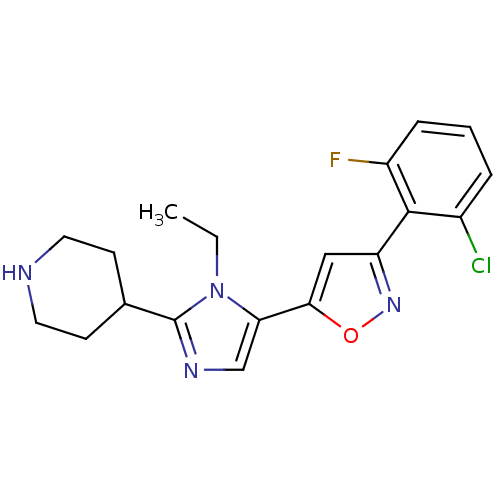
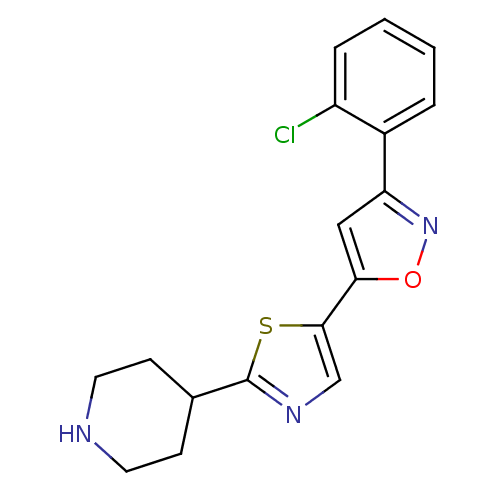
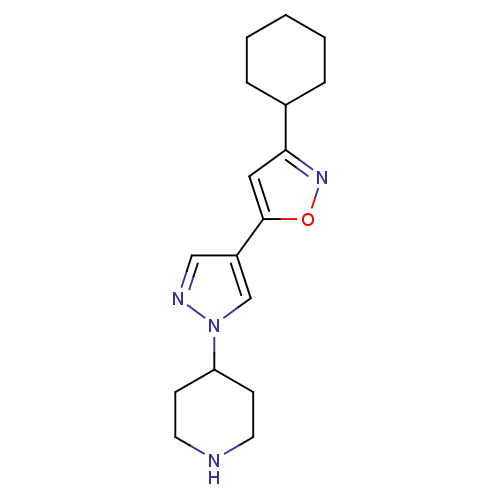
Affinity DataIC50: 120nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+4nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of human beta-secretase (BACE) in MBP-C125 assayMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 3nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta4 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-3(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of human alpha3beta4 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 130nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 34nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 3nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 12nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 33nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4(Homo sapiens (Human))
Amgen Inc.
Curated by ChEMBL
Amgen Inc.
Curated by ChEMBL
Affinity DataEC50: 4.60E+3nMAssay Description:Activation of human alpha4beta2 nAChR assessed as potentiation of submaximal response to nicotine induced currentMore data for this Ligand-Target Pair