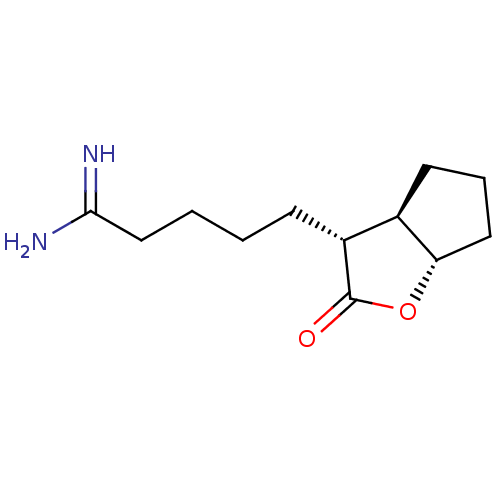
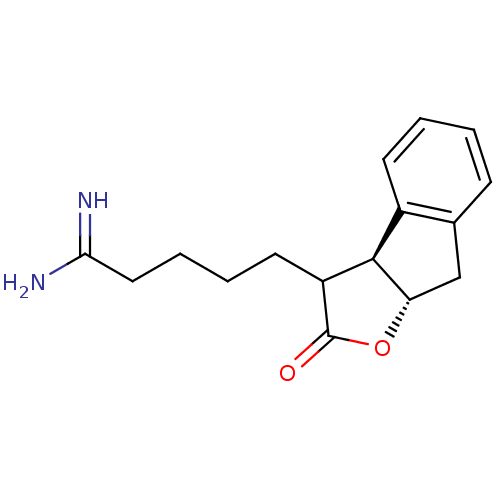
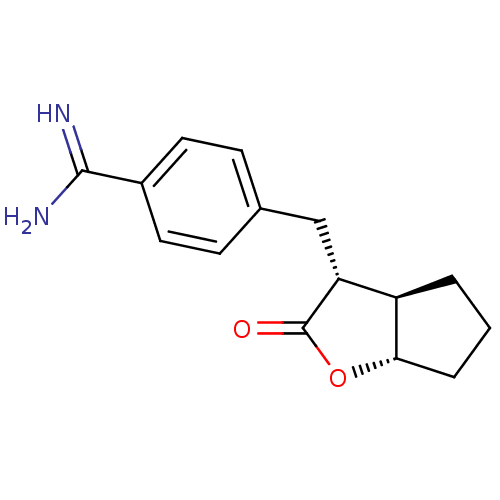
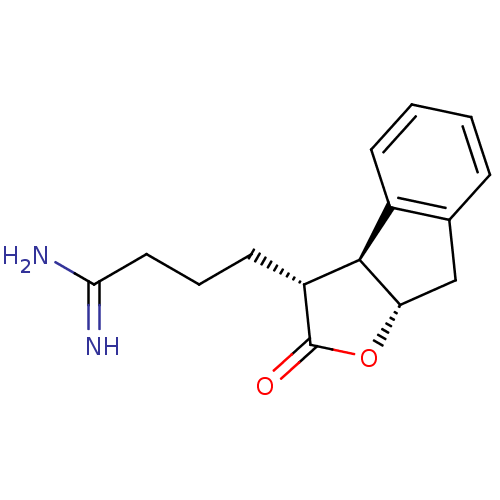
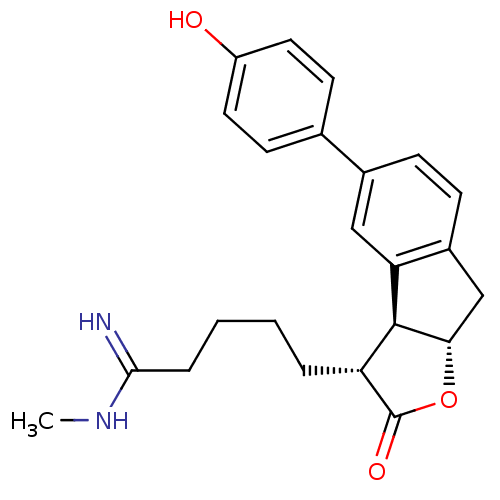
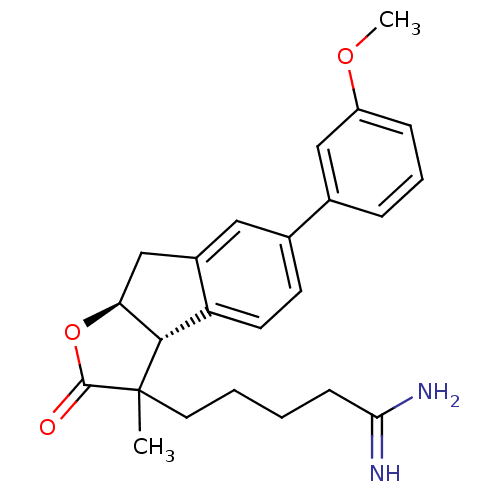
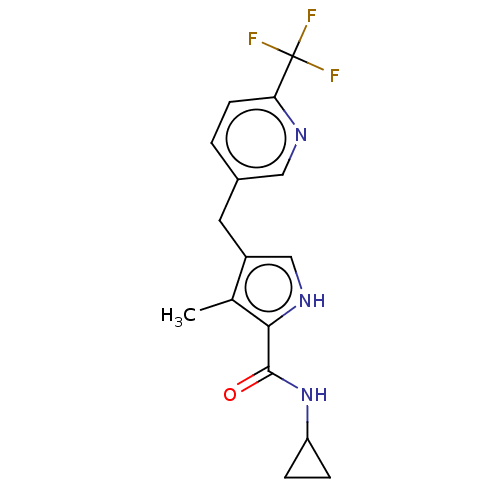
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.120nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Rattus norvegicus (rat))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for the inhibition of Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 77nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Mus musculus)
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Homo sapiens (Human))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of C-terminal His6-tagged human DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibitory activity of the compound against human thrombin was determinedMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Mus musculus)
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assayMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of trypsinMore data for this Ligand-Target Pair
TargetDihydroorotate dehydrogenase (quinone), mitochondrial(Rattus norvegicus (rat))
University of Washington
Curated by ChEMBL
University of Washington
Curated by ChEMBL
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of CYP3A4/5 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition and measured af...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of CYP3A4/5 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by substrate addition and measured...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Compound was evaluated for the inhibition of human leukocyte elastase(HLE).More data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate measured for 10 to 30 mins in presence of NADPH regenerating system by U...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate measured for 10 to 30 mins in presence of NADPH regenerating system by ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate measured for 10 to 30 mins in presence of NADPH regenerating syste...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate measured for 10 to 30 mins in presence of NADPH regenerating syste...More data for this Ligand-Target Pair



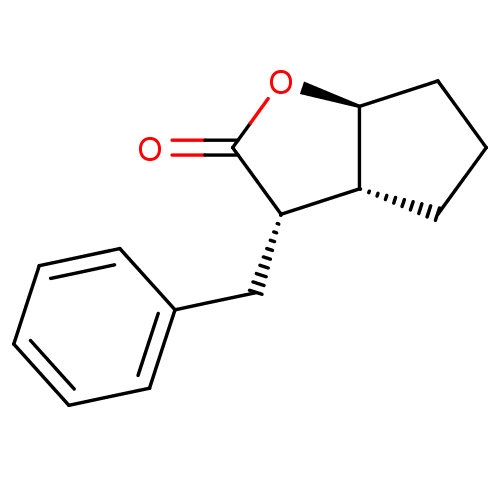
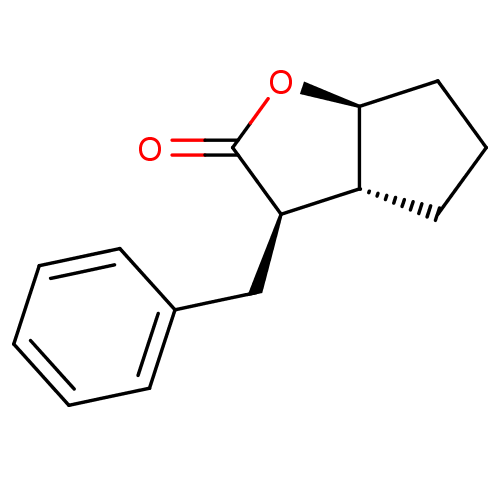


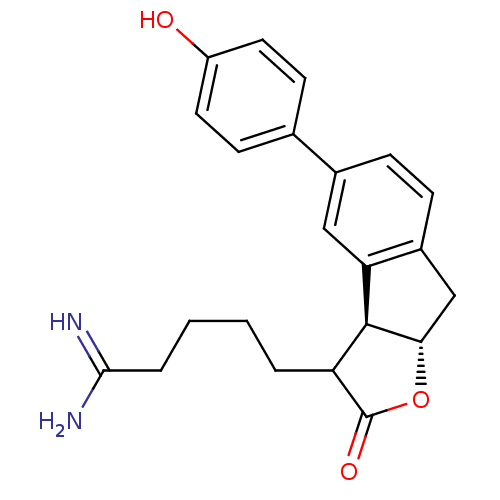
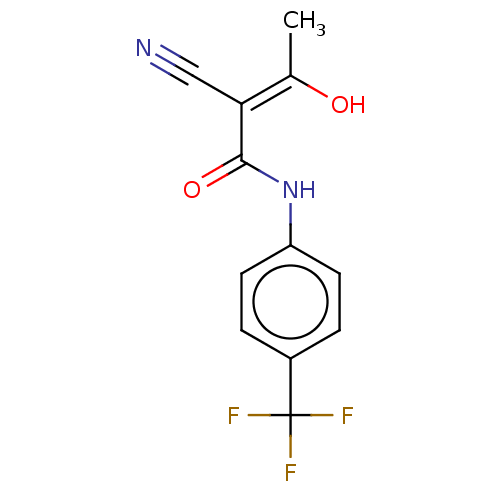
 3D Structure (crystal)
3D Structure (crystal)