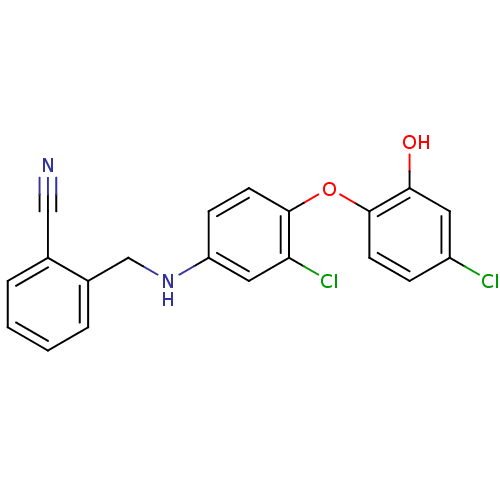
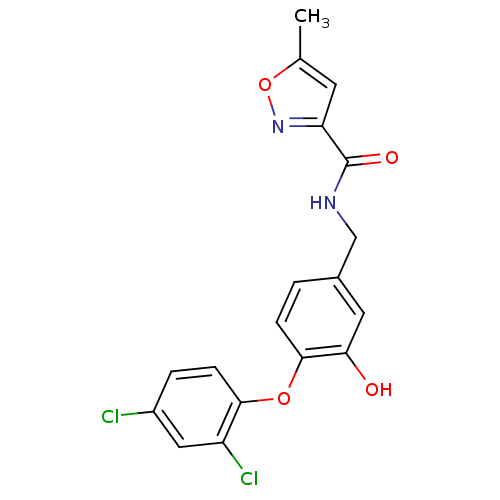
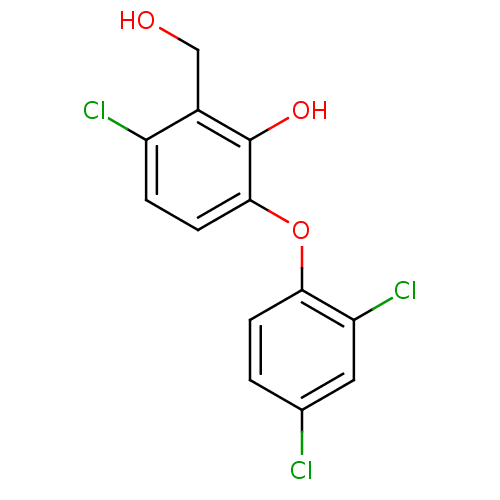
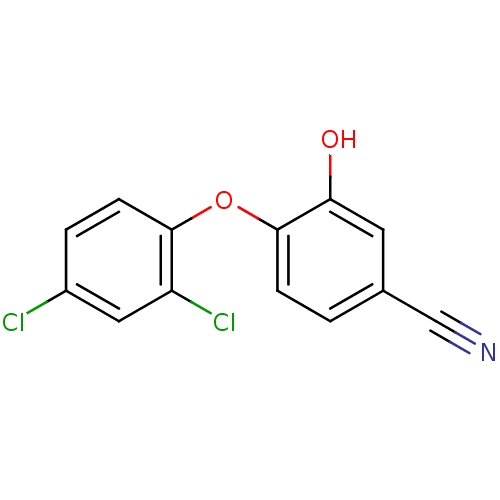
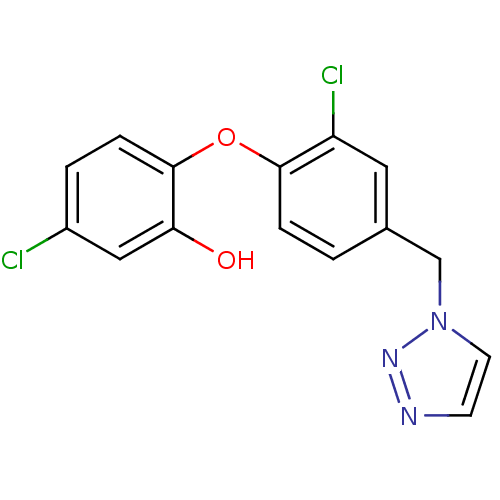
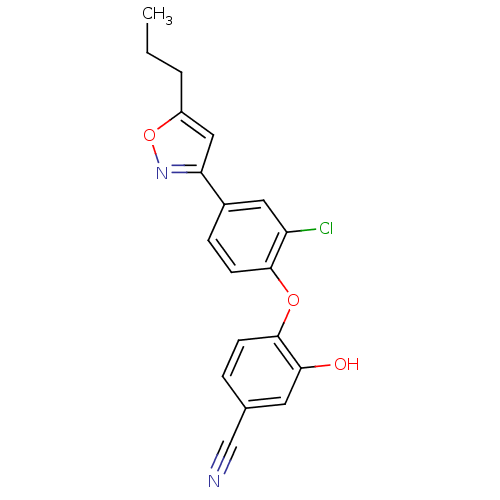
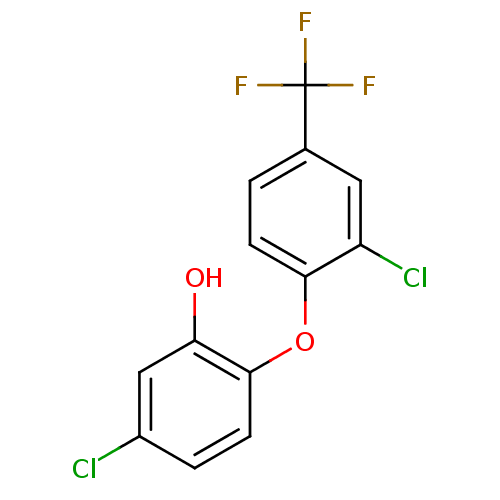
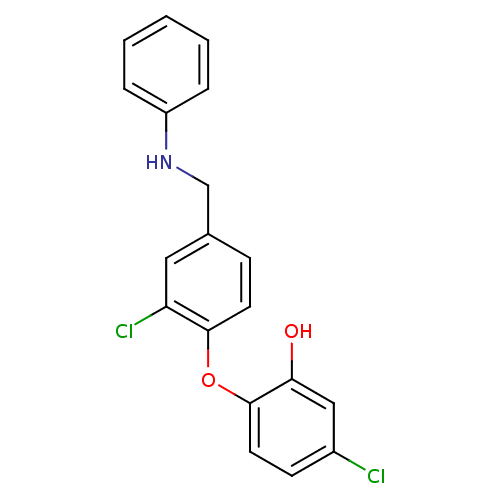
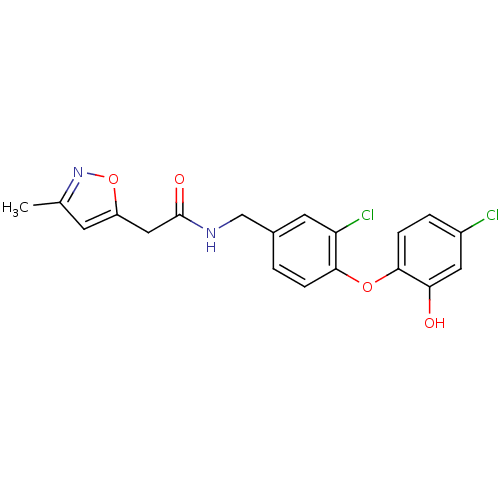
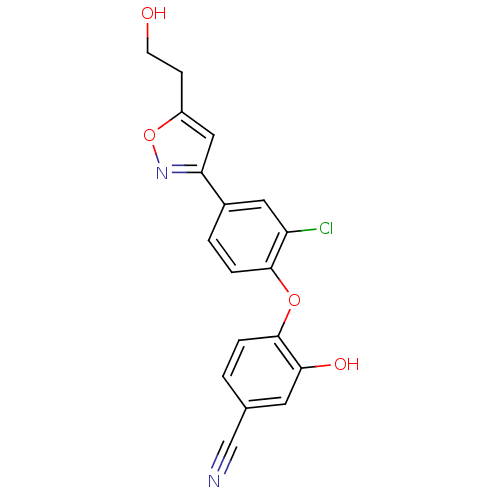
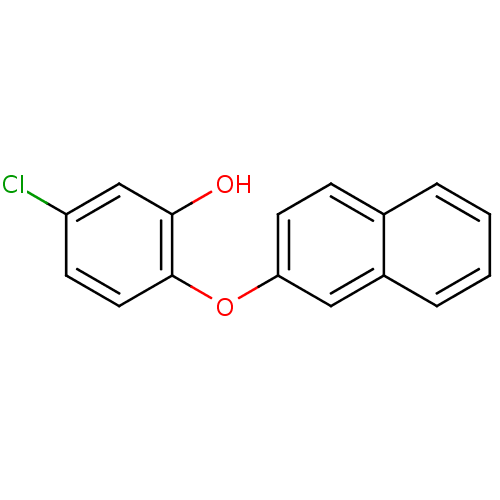
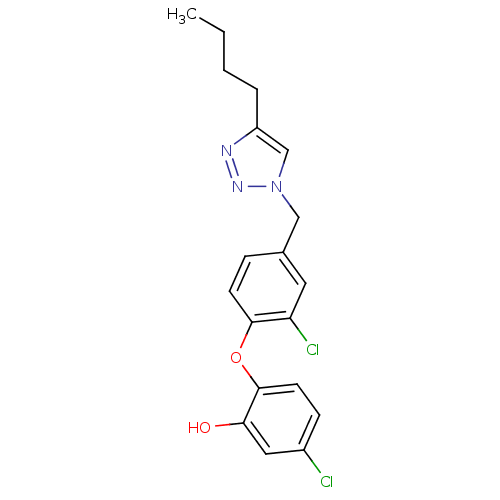
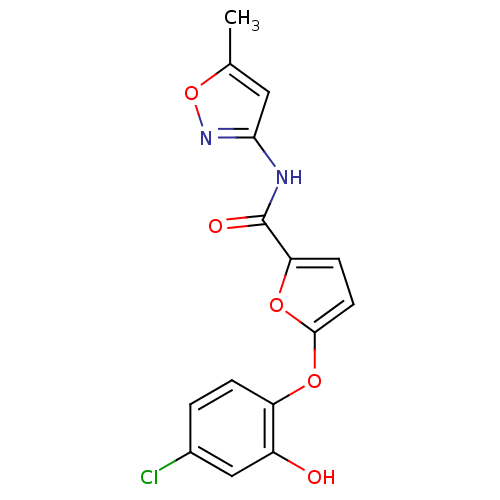
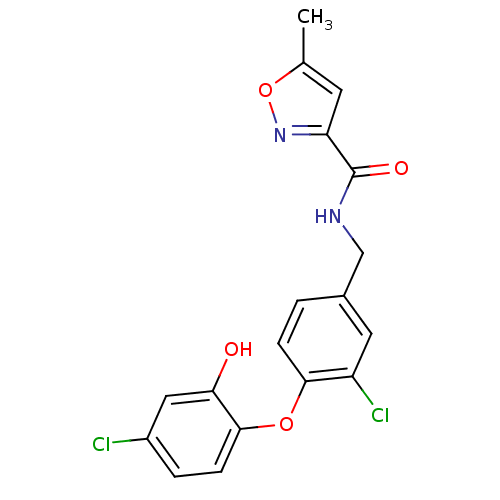
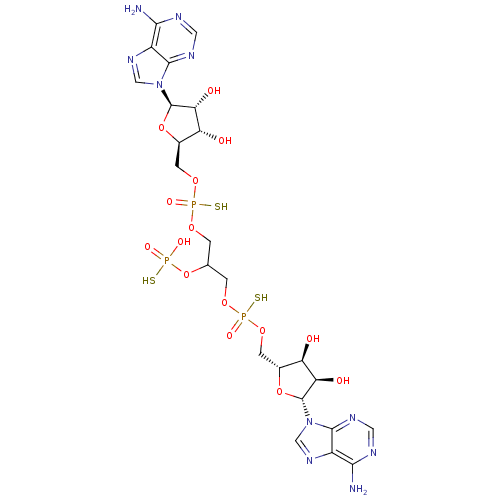
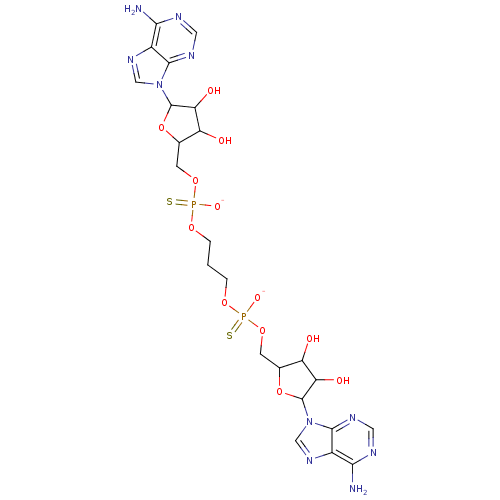
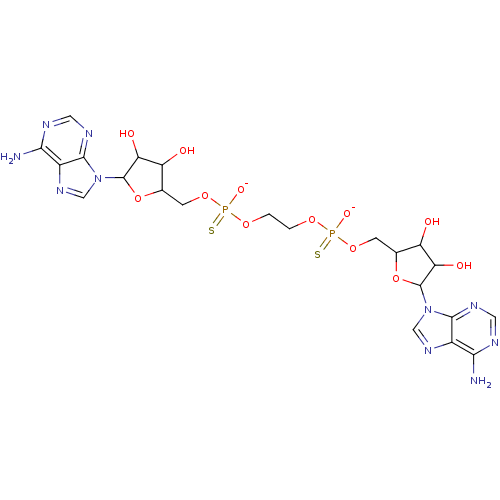
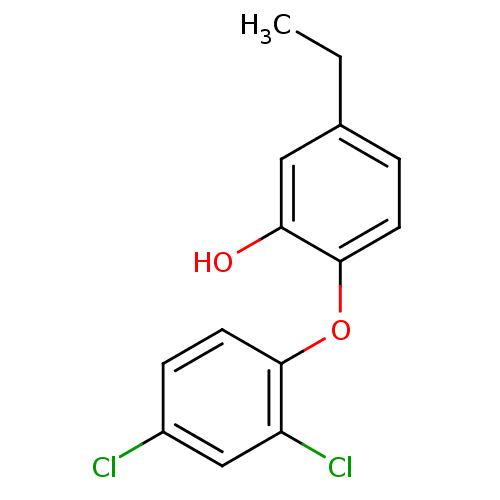
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of Fragile histidine triad prorein hydrolytic activityMore data for this Ligand-Target Pair
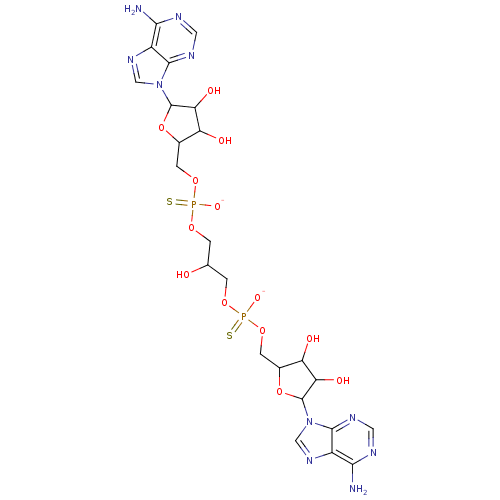
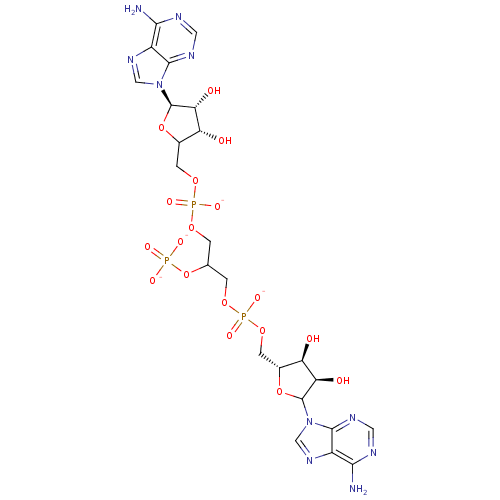
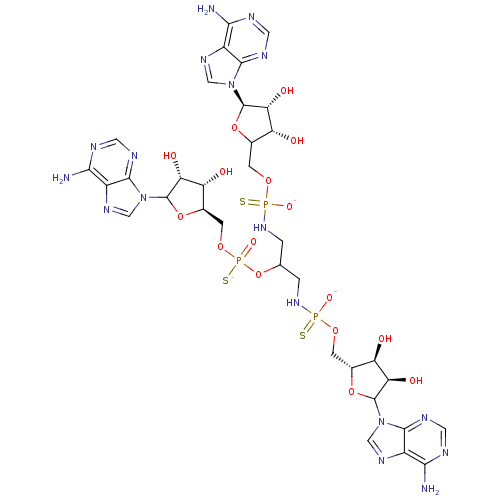
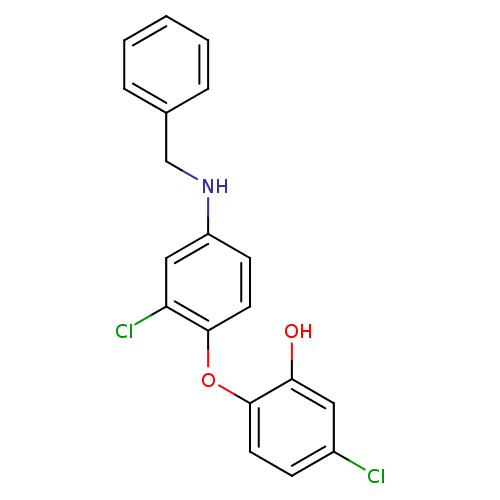
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of Fragile histidine triad prorein hydrolytic activityMore data for this Ligand-Target Pair
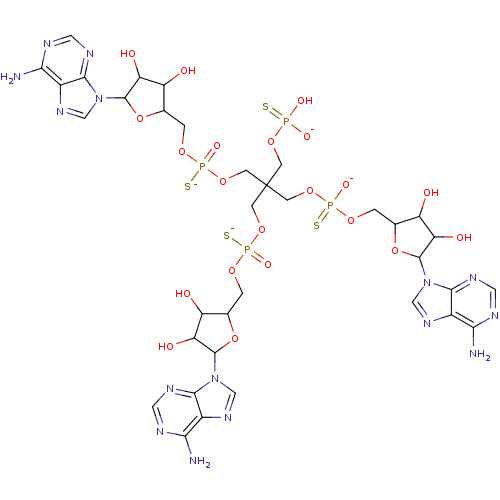
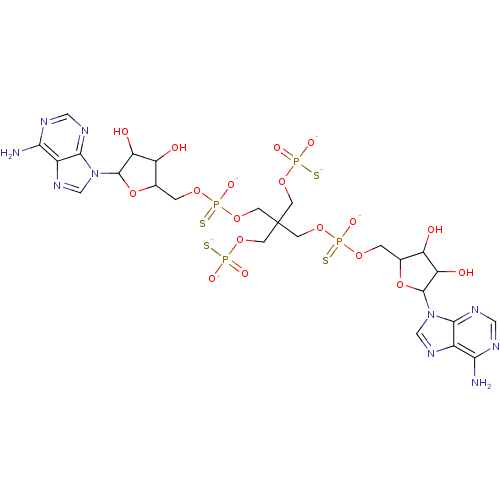
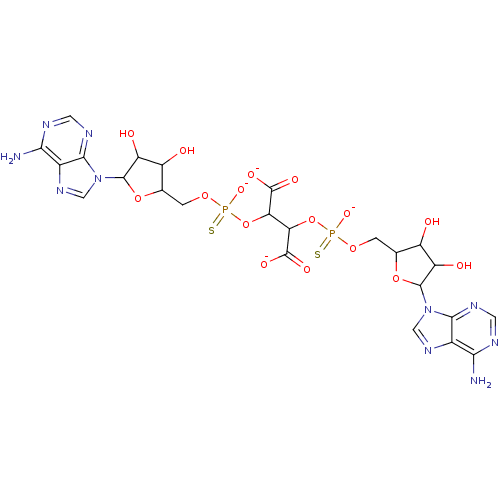
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of Fragile histidine triad prorein hydrolytic activityMore data for this Ligand-Target Pair
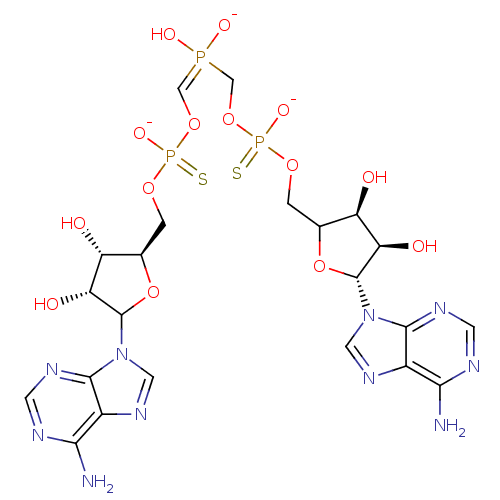
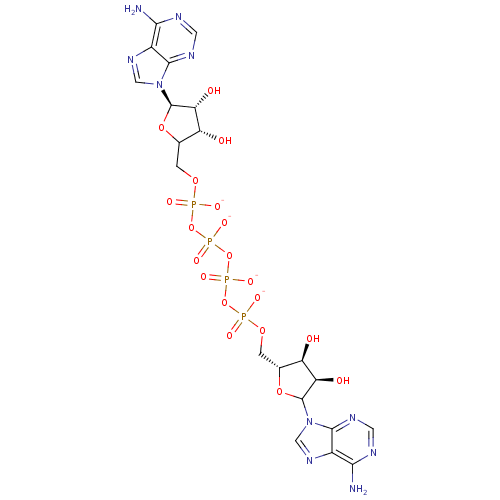
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of Fragile histidine triad prorein hydrolytic activityMore data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of Fragile histidine triad prorein hydrolytic activityMore data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 65nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 78nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 220nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 230nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 420nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 900nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 7.60E+4nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 8.60E+4nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
TargetBis(5'-adenosyl)-triphosphatase(Homo sapiens (Human))
Polish Academy of Sciences
Curated by ChEMBL
Polish Academy of Sciences
Curated by ChEMBL
Affinity DataKi: 8.60E+4nMAssay Description:Inhibition assay using Fhit with ApppBODIPY.More data for this Ligand-Target Pair
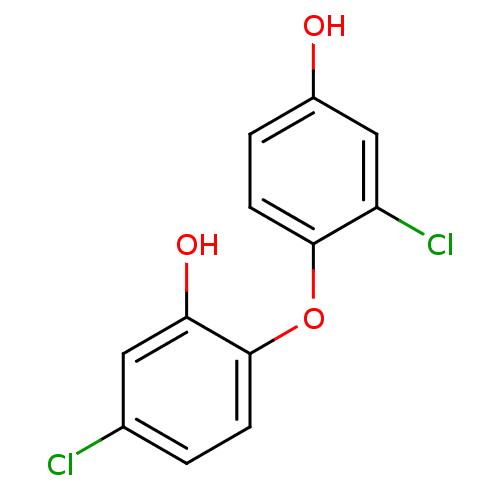
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 3nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 8nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 13nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 15nMAssay Description:Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 15nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 16nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 18nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 19nMAssay Description:Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 19nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 23nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 24nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 26nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 29nMAssay Description:Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 30nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 31nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 33nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 34nMAssay Description:Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 41nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 43nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 58nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
Johns Hopkins Bloomberg School of Public Health
Johns Hopkins Bloomberg School of Public Health
Affinity DataIC50: 100nMpH: 7.5Assay Description:The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot...More data for this Ligand-Target Pair


















 3D Structure (crystal)
3D Structure (crystal)