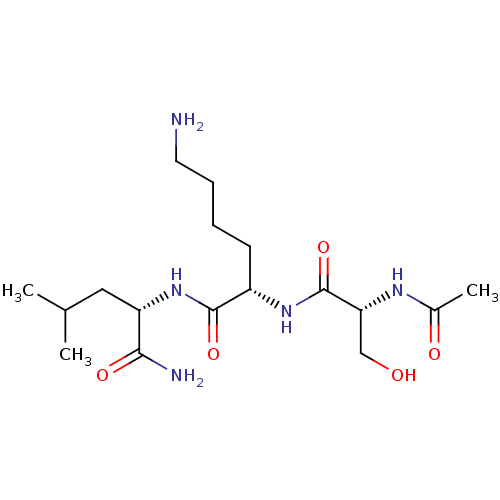
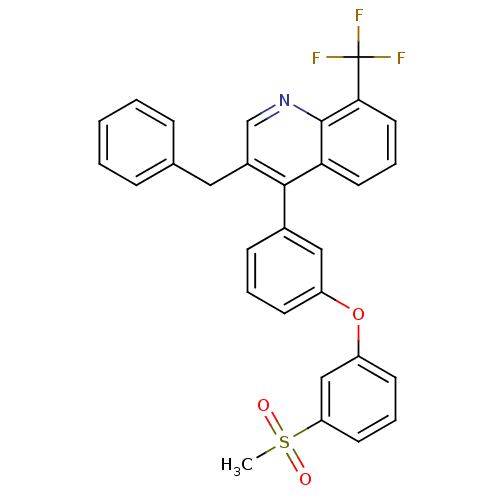
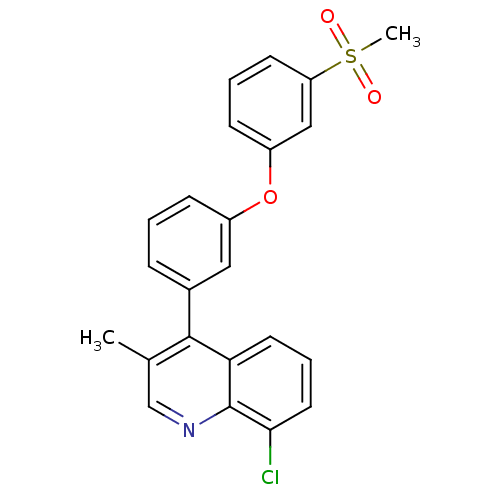
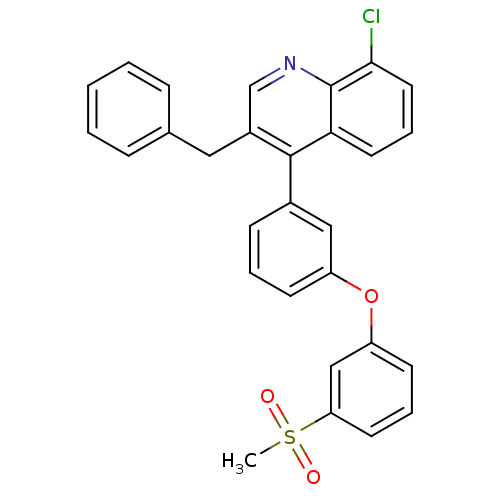
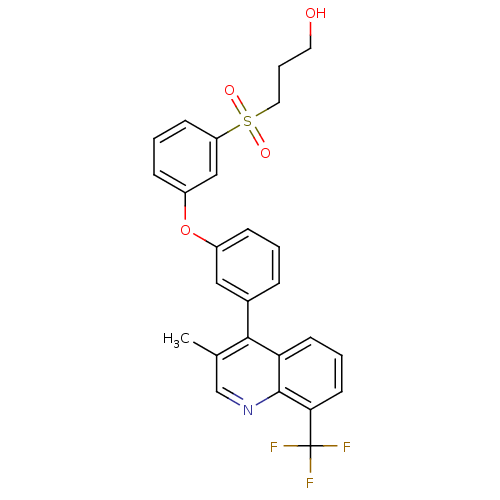
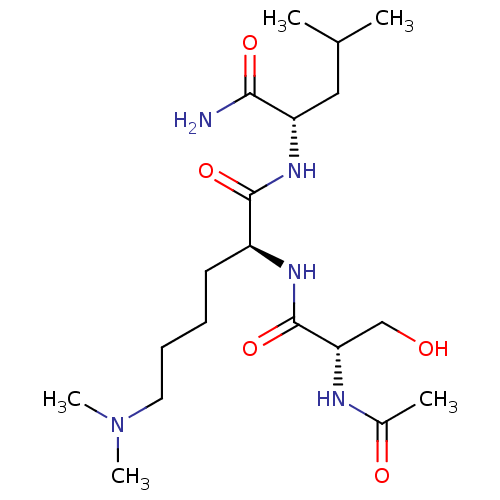
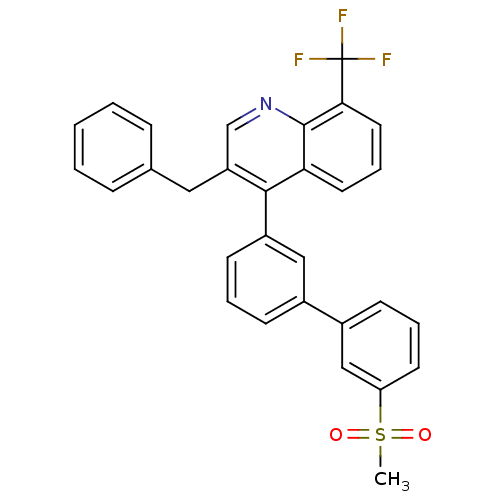
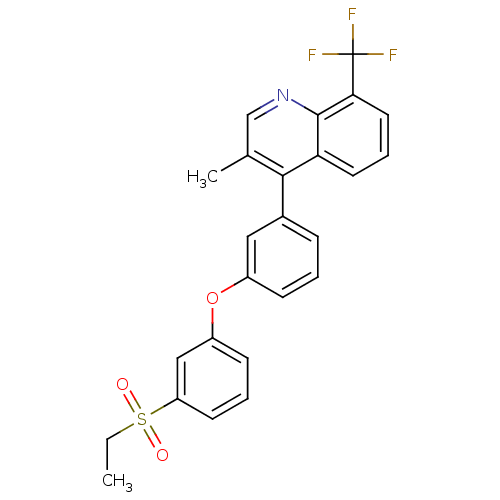
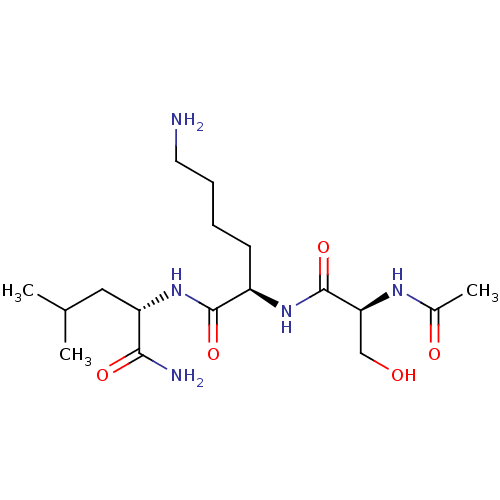
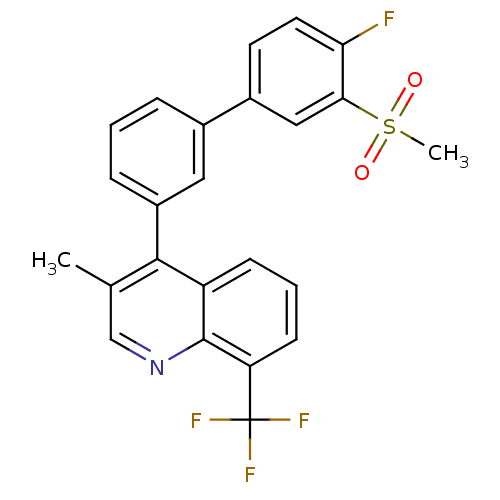
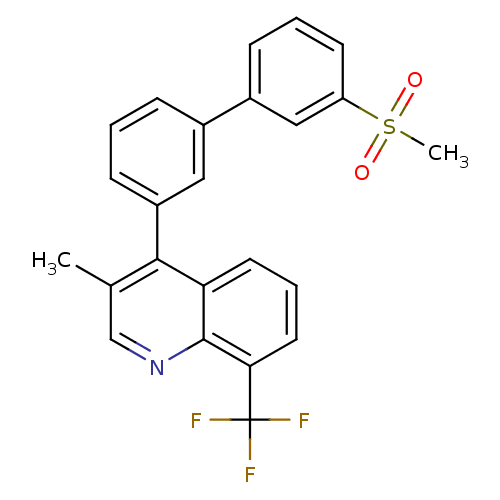
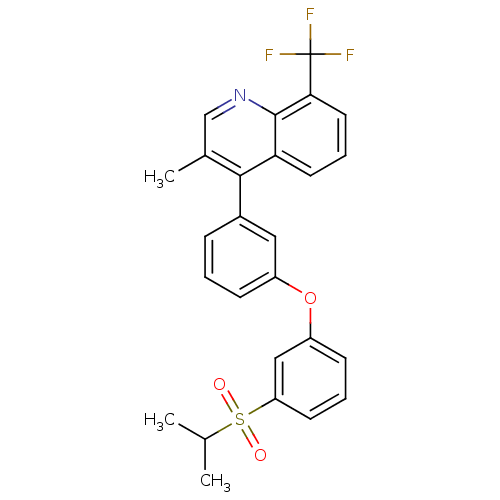
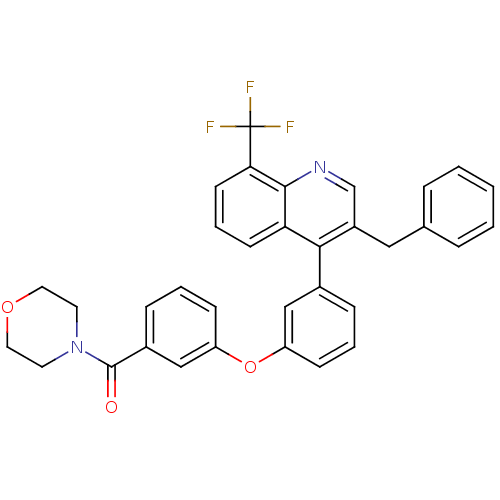
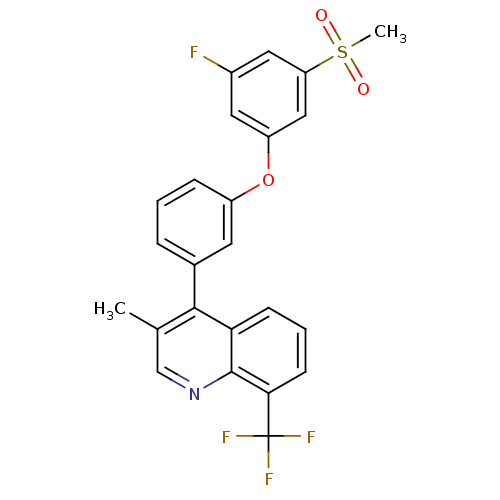
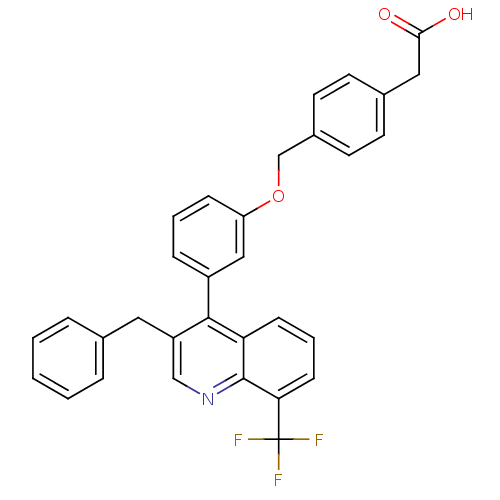
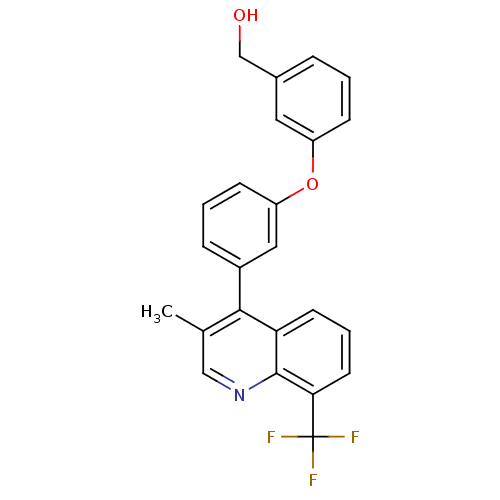
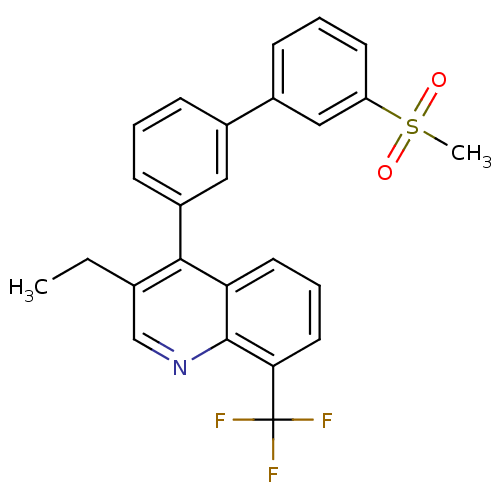
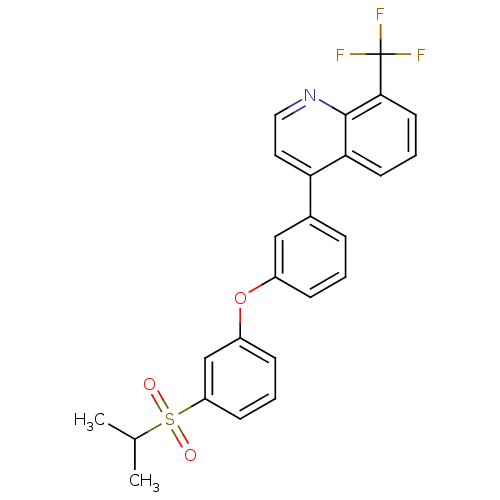
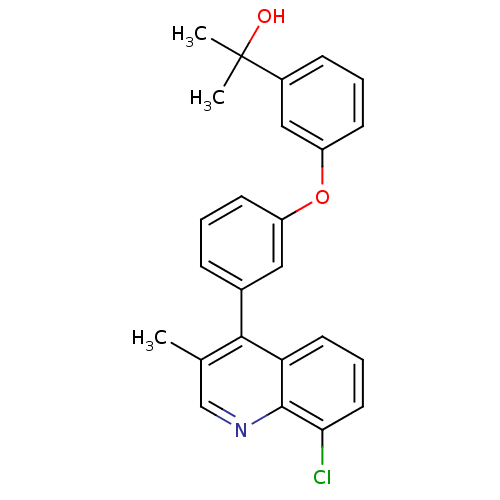
Affinity DataKi: 10nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of matrix metalloproteinase-2 (MMP-2), gelatinase-AMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of matrix metalloproteinase-1 (MMP-1), fibroblast collagenase.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of matrix metalloproteinase-3 (MMP-3), stromelysin-1More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of [3H]T0901317 from LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]T0901317 from LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Displacement of [3H]T0901317 from LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nM EC50: 138nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 93nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 233nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nM EC50: 71nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of TSP1 in human myeloma cells assessed reduction in TGFbeta activity incubated for 1 hr by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nM EC50: 108nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair

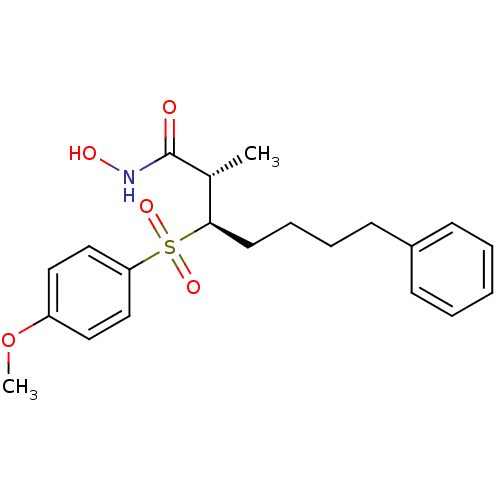
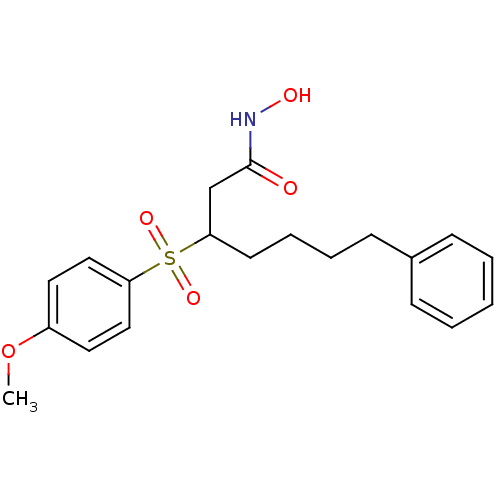
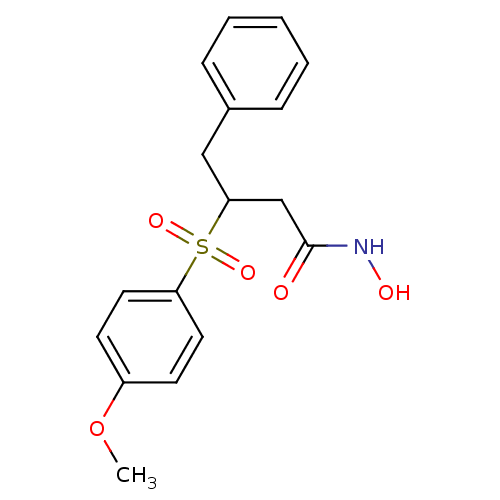
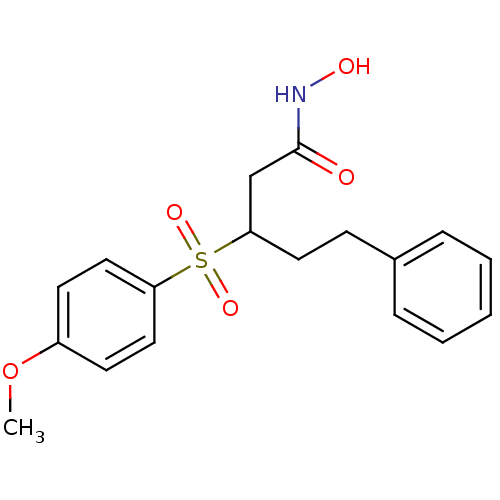
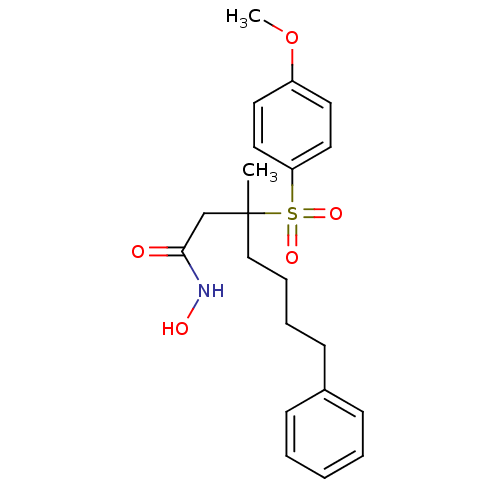
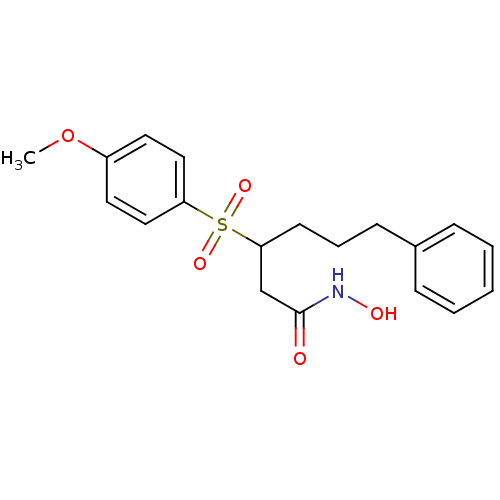
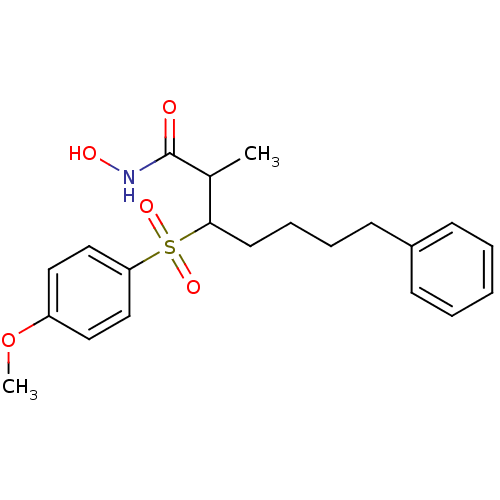
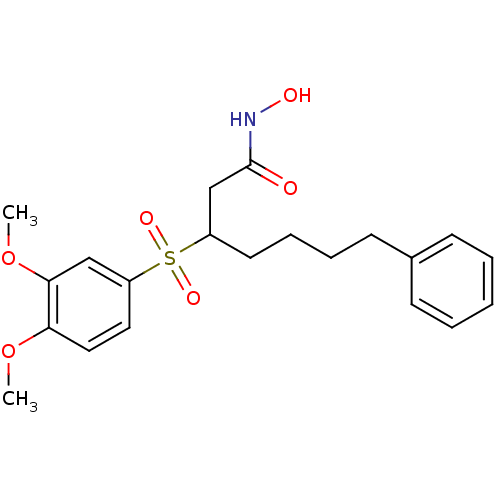

 3D Structure (crystal)
3D Structure (crystal)