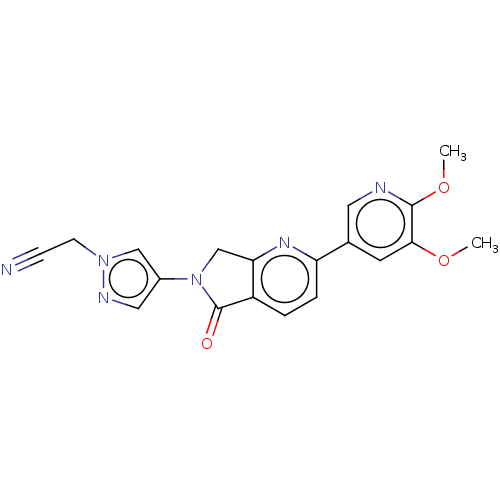
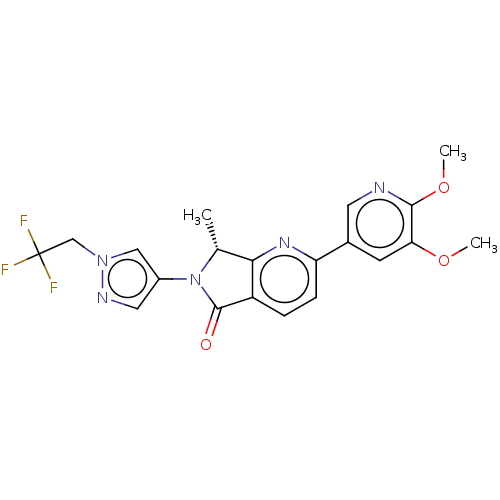
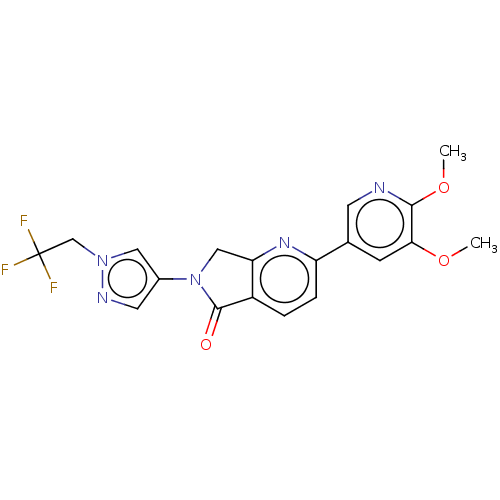
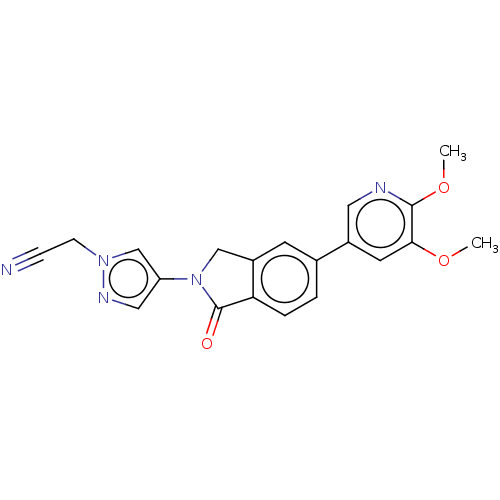
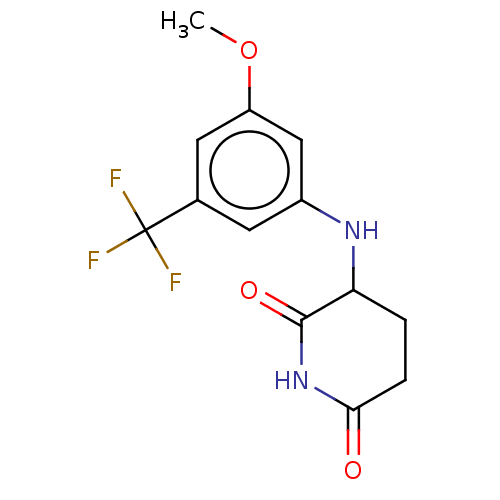
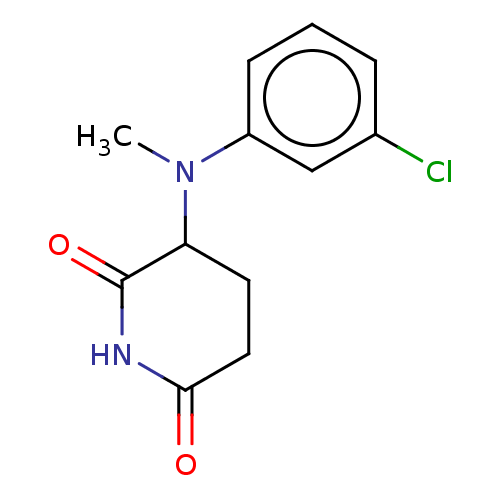
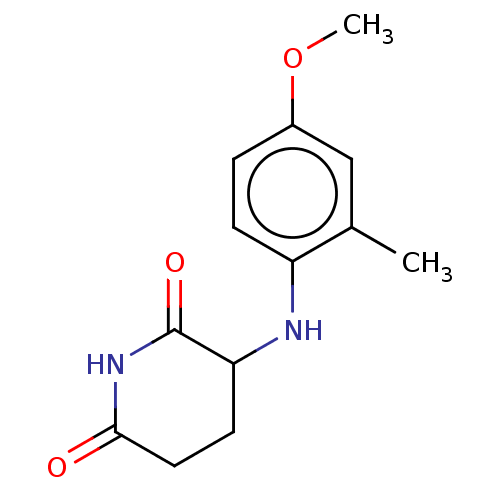
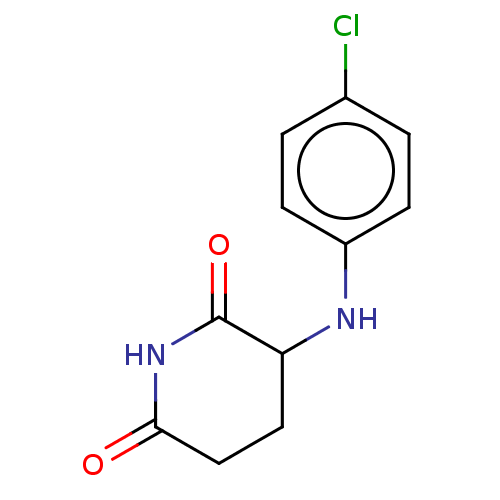
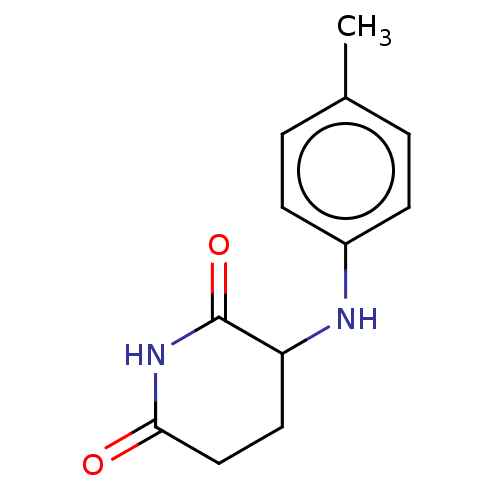
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
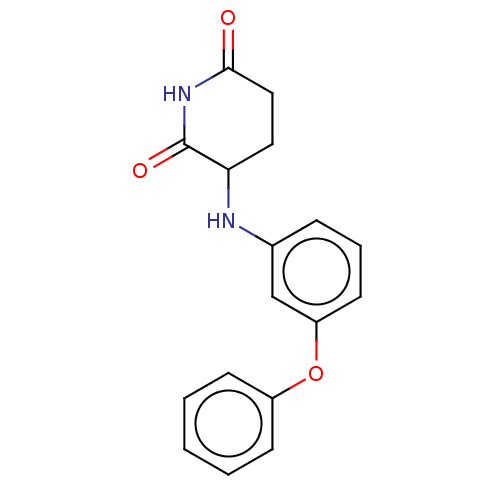
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
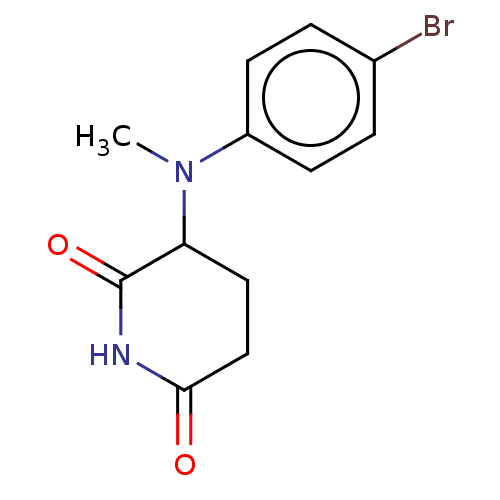
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
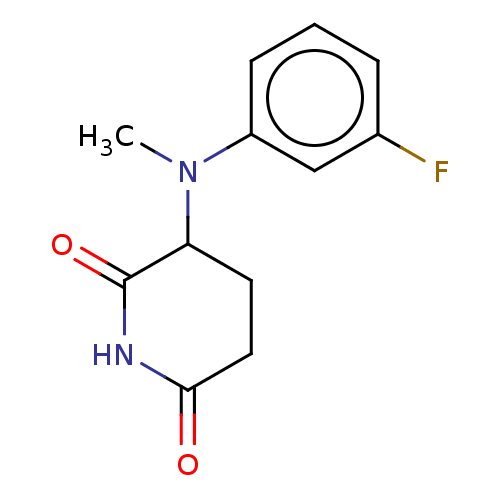
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDNA-dependent protein kinase catalytic subunit(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Inhibition of DNA-PK (unknown origin) using EPPLSQEAFADLWKKK as substrate after 15 mins in presence of [33P-ATP] by radiometric methodMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of PI3Kgamma in TNFalpha primed human neutrophils assessed as inhibition of fMLP-induced ROS generation preincubated for 30 to 60 mins fol...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow...More data for this Ligand-Target Pair
TargetPhosphatidylinositol-4,5-bisphosphate 3-kinase(Rattus norvegicus)
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of PI3Kgamma in rat spleenocytes assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of PI3Kgamma in human THP1 cells assessed as inhibition of MCP1-induced Akt phosphorylation at Ser-47 residue preincubated for 1 hr follow...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Vertex Pharmaceuticals Inc
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of PI3Kgamma in TNFalpha primed human whole blood assessed as inhibition of fMLP-induced ROS generation preincubated for 30 to 60 mins fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 2.51E+4nMAssay Description:Inhibition of human ERG by Q-patch assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG channel by planar techniqueMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG channel by QPatch techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human ERG by Q-patch assayMore data for this Ligand-Target Pair

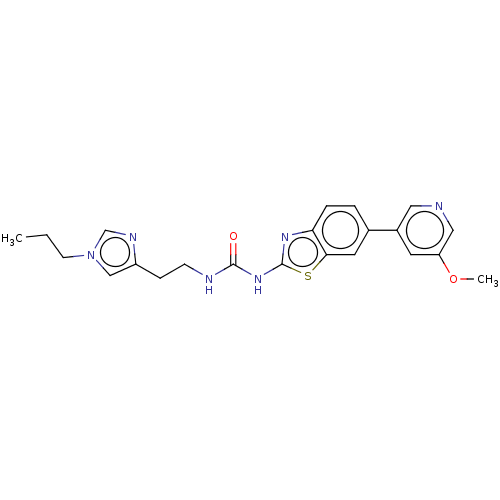
 3D Structure (crystal)
3D Structure (crystal)