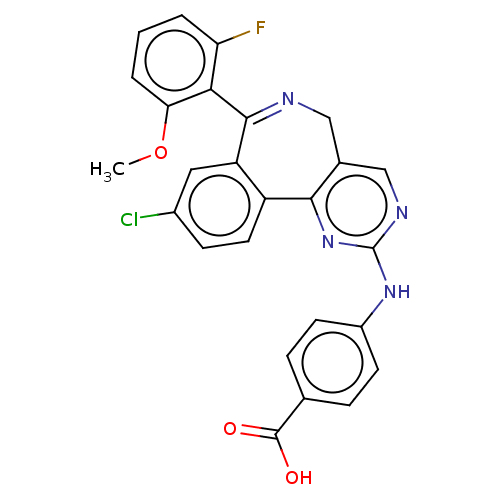
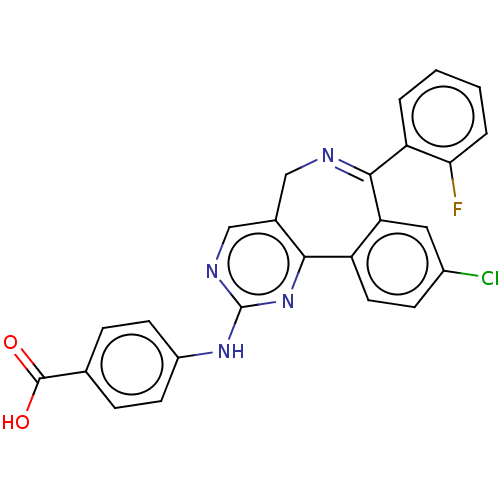
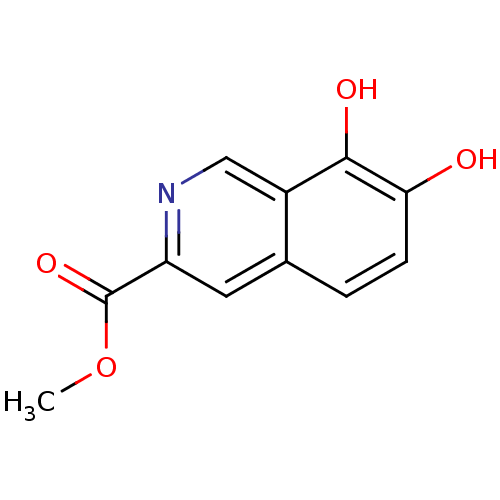
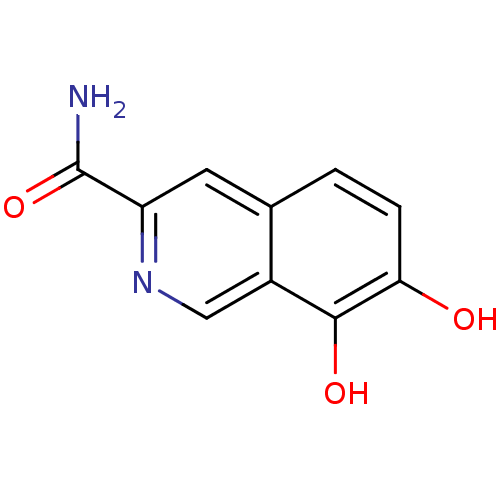
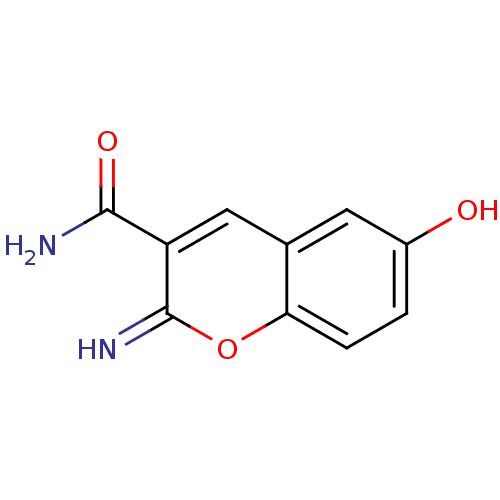
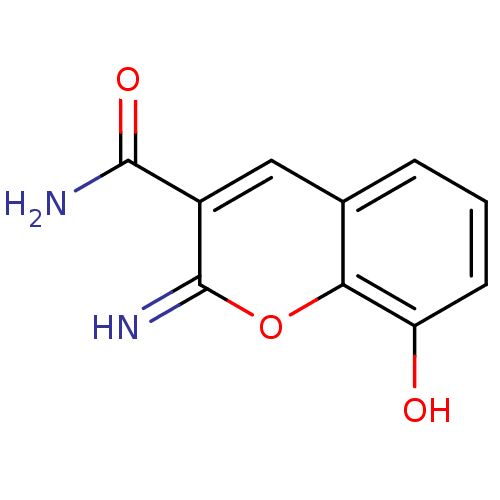
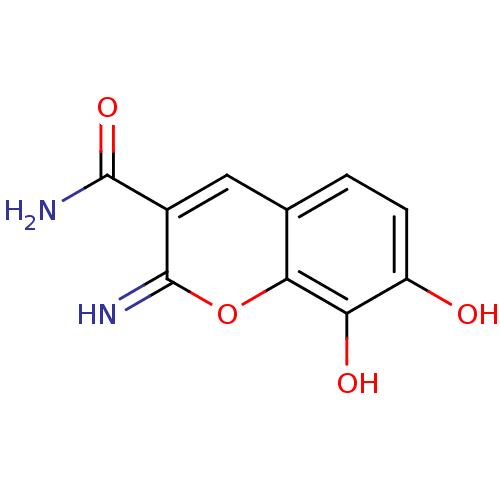
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
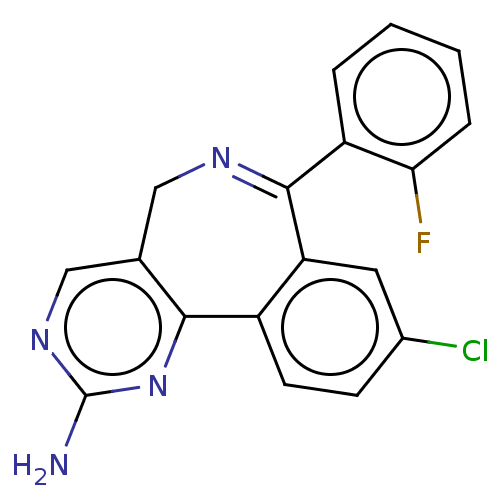
Affinity DataKi: 0.300nMAssay Description:Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of mouse recombinant Aurora A kinase expressed in insect Sf9 cells by radioactive flashplate assayMore data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
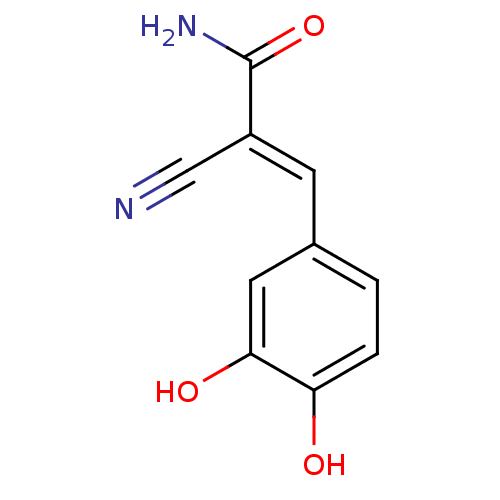
Affinity DataIC50: 7nMAssay Description:Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
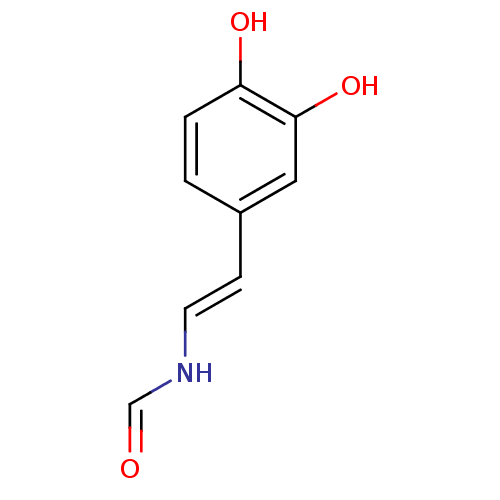
Affinity DataIC50: 34nMAssay Description:Inhibition of Aurora A Thr288 autophosphorylation in human HeLa cells after 1 hrMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
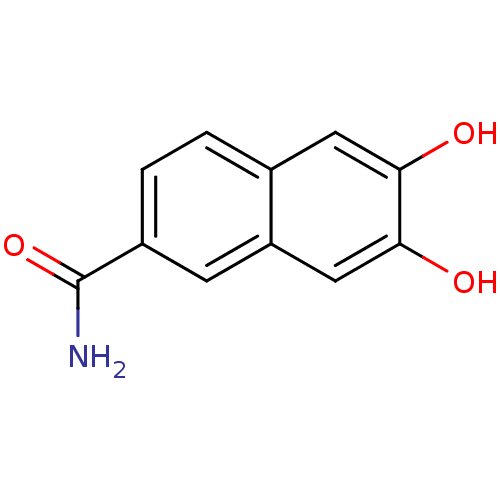
Affinity DataIC50: 150nMAssay Description:Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin)More data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 172nMAssay Description:Inhibition of mouse recombinant Aurora B kinase expressed in insect Sf9 cells by radioactive flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin)More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetAurora kinase A(Mus musculus (mouse))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATPMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of LCK by radioactive flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 5.20E+3nMAssay Description:Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of Aurora B Ser10 phosphorylation in human HeLa cells after 1 hrMore data for this Ligand-Target Pair
TargetAurora kinase A(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of p56 lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
TargetAurora kinase B(Homo sapiens (Human))
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States.
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of aurora kinase B in human HCT116 cells assessed as inhibition of histone H3 phosphorylation by immunofluorescence analysisMore data for this Ligand-Target Pair
TargetCasein kinase II subunit alpha(Homo sapiens (Human))
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 2.05E+4nMAssay Description:Inhibition of CK2 by radioactive flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+4nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk2(Homo sapiens (Human))
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of CHK2 by radioactive flashplate assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 5.30E+4nMAssay Description:Inhibition of PLK1 by radioactive flashplate assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CDK1 by radioactive flashplate assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CHK1 by radioactive flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of p56 lck tyrosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of insulin receptor autophosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation in A431 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+5nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+5nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Ability to inhibit autophosphorylation of immunopurified p56IckMore data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)